Unit 7 Thermochemistry Calculations Phase Change and qmcT


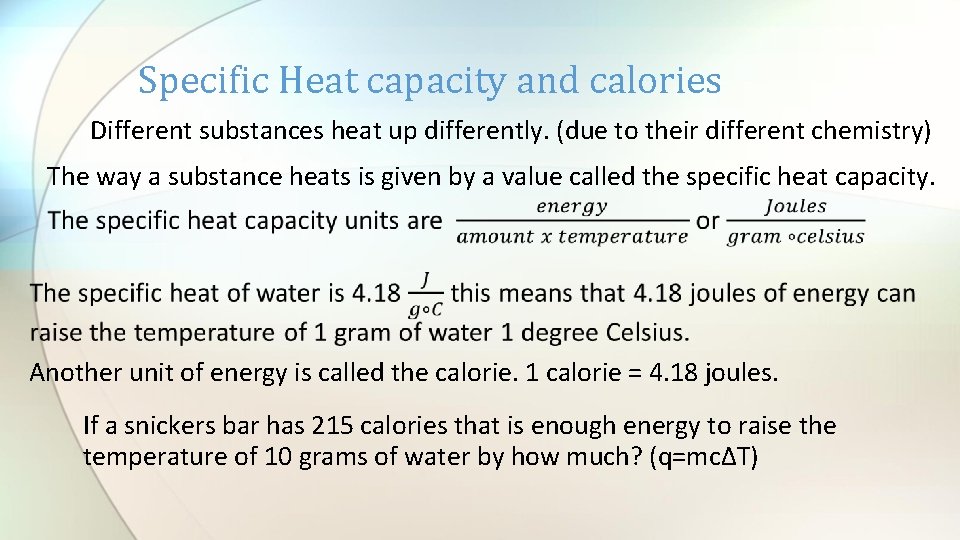
- Slides: 4

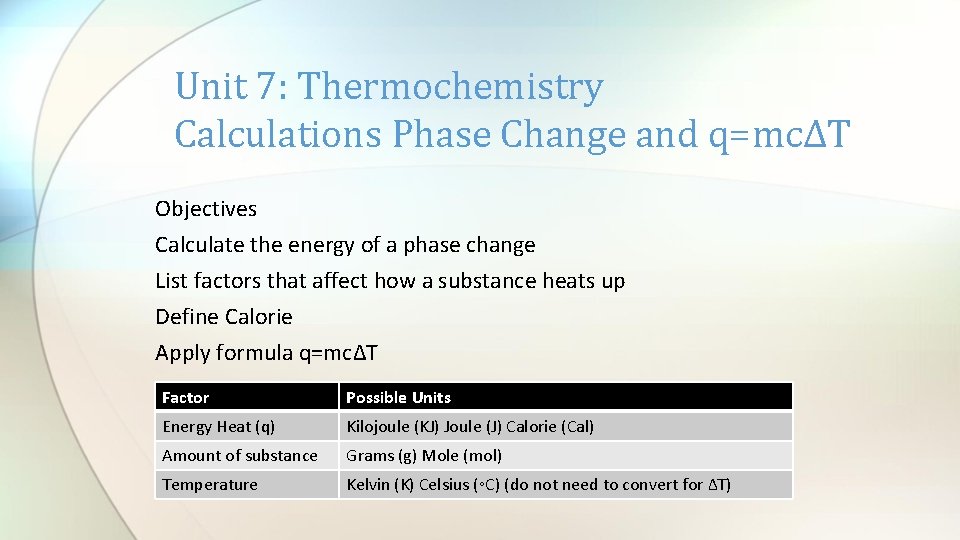
Unit 7: Thermochemistry Calculations Phase Change and q=mcΔT Objectives Calculate the energy of a phase change List factors that affect how a substance heats up Define Calorie Apply formula q=mcΔT Factor Possible Units Energy Heat (q) Kilojoule (KJ) Joule (J) Calorie (Cal) Amount of substance Grams (g) Mole (mol) Temperature Kelvin (K) Celsius (◦C) (do not need to convert for ∆T)

Heat of Fusion and Vaporization ΔHfus = Heat of fusion = Energy change in kilojoules/Mole when a substance freezes or melts. (Solid/Liquid) ΔHvap = Heat of vaporization = Energy change in Kilojoules/Mole when a substance boils (vaporizes) or condenses. (Liquid/Gas) The standard heat of fusion of water = 6. 02 KJ/Mole This means it takes 6. 02 KJ to change 1 mole of water into ice or 1 mole of ice into water. The sign depends on which way the question is asking. Given that a box of chocolates costs $6. 02/box how much would 3 boxes cost? Given 3 moles of water are frozen to ice what is the energy change in KJ? https: //www. youtube. com/watch? v=6 Bzgd 5 M-t 4 U

Factors for Heating. The amount of heat (q) it takes to heat up a substance depends on a variety of factors. Determine which takes more energy for the following. Two cups of water, cup 1 and cup 2, everything is equal except cup 1 will be heated to 40 ◦ C and cup 2 will be heated to 80 ◦ C. Which takes more energy? A cup of water and a bucket of water. Both are being heated to 40◦ C. A beaker of water and a beaker of mineral oil. Both are being heated to 35 ◦ C So what factors determine heating? What is the formula
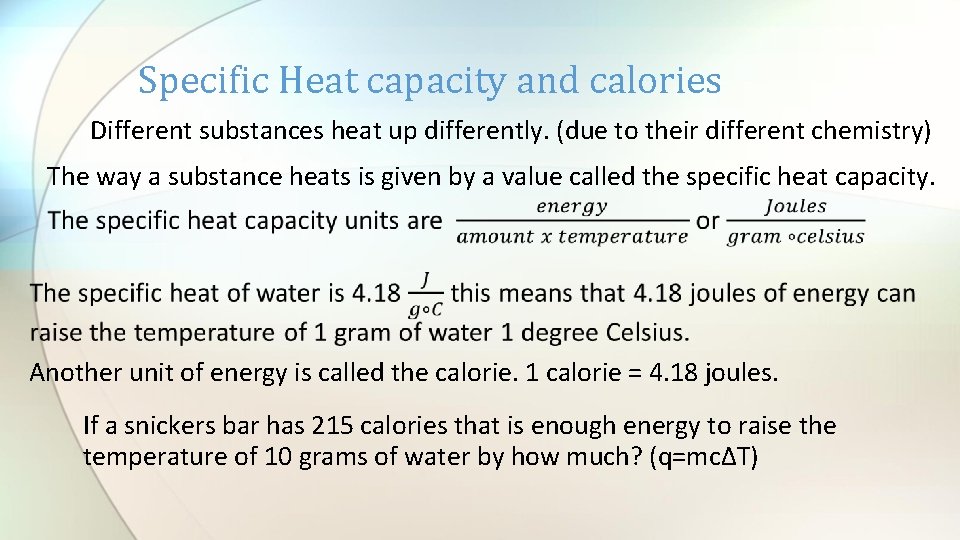
Specific Heat capacity and calories Different substances heat up differently. (due to their different chemistry) The way a substance heats is given by a value called the specific heat capacity. Another unit of energy is called the calorie. 1 calorie = 4. 18 joules. If a snickers bar has 215 calories that is enough energy to raise the temperature of 10 grams of water by how much? (q=mcΔT)