UNIT 7 The Mole PERCENT COMPOSITION EMPIRICAL AND







- Slides: 21

UNIT 7 The Mole PERCENT COMPOSITION EMPIRICAL AND MOLECULAR FORMULAS

PERCENT COMPOSITION

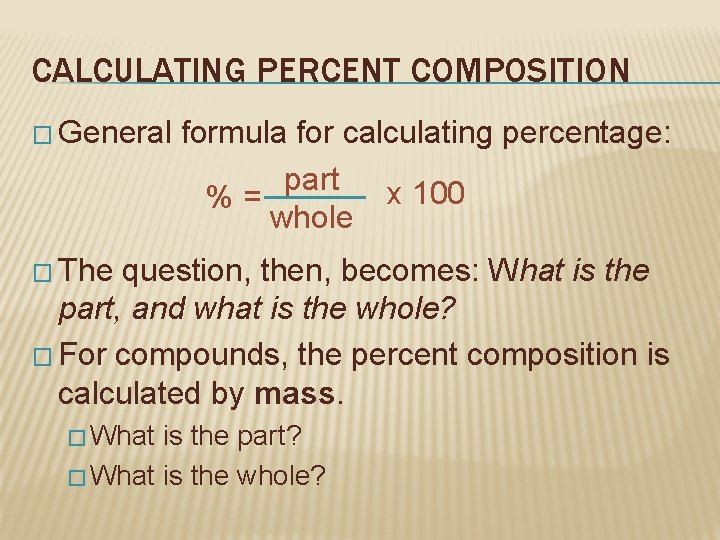
CALCULATING PERCENT COMPOSITION � General formula for calculating percentage: part x 100 %= whole � The question, then, becomes: What is the part, and what is the whole? � For compounds, the percent composition is calculated by mass. � What is the part? � What is the whole?

UNKNOWN COMPOUND � Recently discovered new compound � Named cookie � Known to be comprised of two elements, Cc and Do, in an unknown ratio � Chocolate � Task 1. 2. chip and cookie dough, respectively is two-fold: Calculate the percent composition by mass Identify empirical formula of compound � Empirical formula indicates the simplest whole number ratio of atoms present in compound

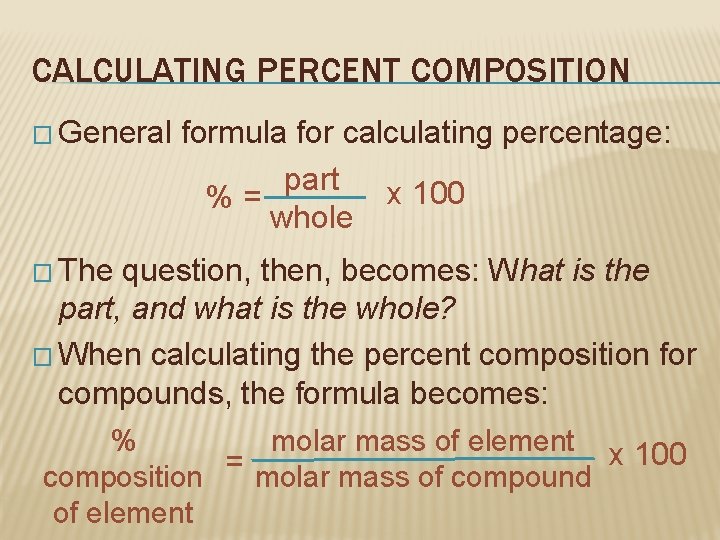
CALCULATING PERCENT COMPOSITION � General formula for calculating percentage: part x 100 %= whole � The question, then, becomes: What is the part, and what is the whole? � When calculating the percent composition for compounds, the formula becomes: % molar mass of element x 100 composition = molar mass of compound of element

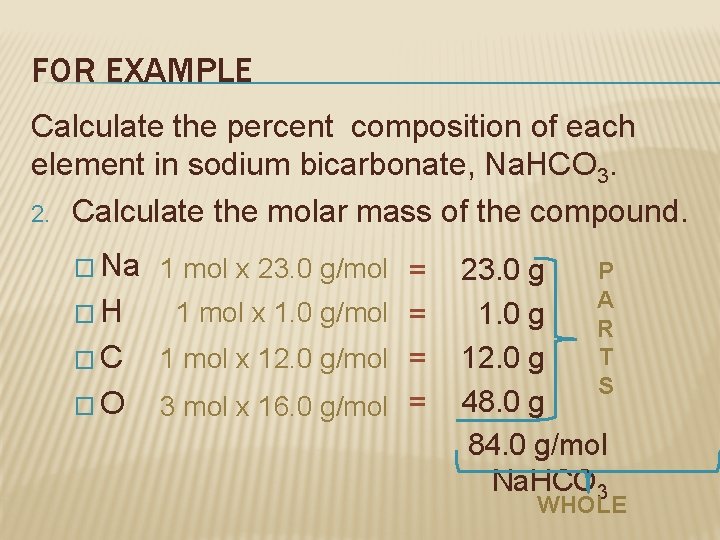
FOR EXAMPLE Calculate the percent composition of each element in sodium bicarbonate (sodium hydrogen carbonate). 1. Write the formula for sodium bicarbonate. 1– Na 1+Na. HCOHCO 3 3

FOR EXAMPLE Calculate the percent composition of each element in sodium bicarbonate, Na. HCO 3. 2. Calculate the molar mass of the compound. � Na 1 mol x 23. 0 g/mol = �H 1 mol x 1. 0 g/mol = �C 1 mol x 12. 0 g/mol = �O 3 mol x 16. 0 g/mol = P 23. 0 g A 1. 0 g R T 12. 0 g S 48. 0 g 84. 0 g/mol Na. HCO 3 WHOLE

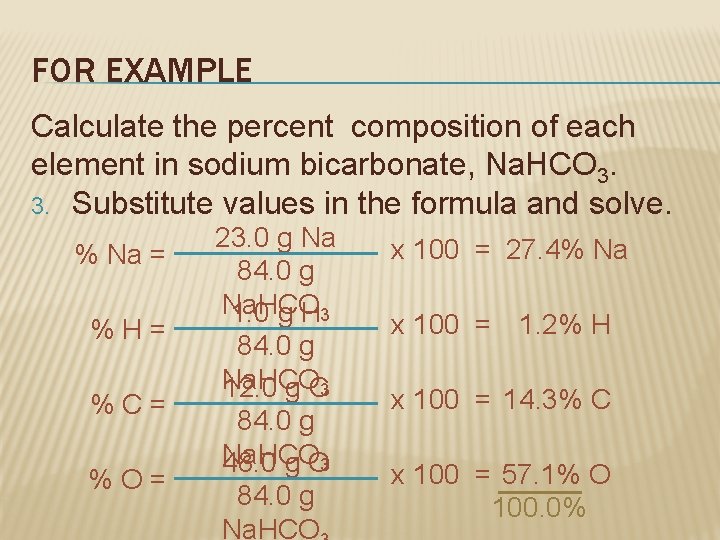
FOR EXAMPLE Calculate the percent composition of each element in sodium bicarbonate, Na. HCO 3. 3. Substitute values in the formula and solve. % Na = %H= %C= %O= 23. 0 g Na 84. 0 g Na. HCO 1. 0 g H 3 84. 0 g Na. HCO 12. 0 g C 3 84. 0 g Na. HCO 48. 0 g O 3 84. 0 g Na. HCO x 100 = 27. 4% Na x 100 = 1. 2% H x 100 = 14. 3% C x 100 = 57. 1% O 100. 0%

EMPIRICAL AND MOLECULAR FORMULAS

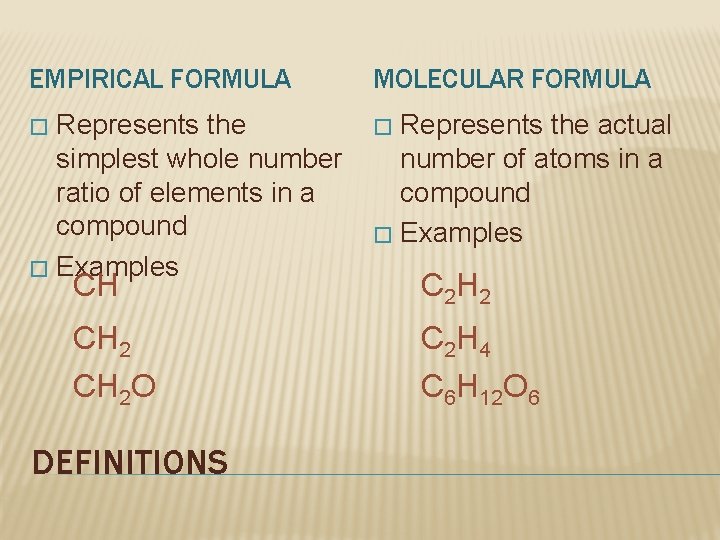
EMPIRICAL FORMULA MOLECULAR FORMULA Represents the simplest whole number ratio of elements in a compound � Examples � � Represents the actual number of atoms in a compound � Examples CH C 2 H 2 CH 2 O C 2 H 4 C 6 H 12 O 6 DEFINITIONS

EMPIRICAL FORMULA

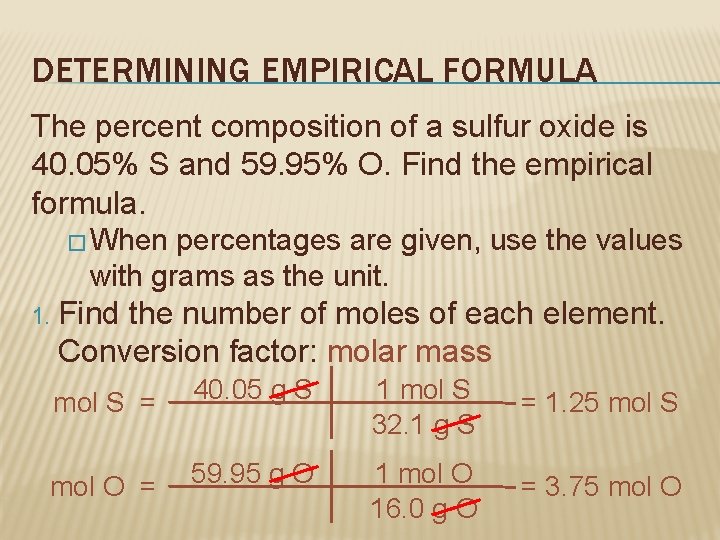
DETERMINING EMPIRICAL FORMULA The percent composition of a sulfur oxide is 40. 05% S and 59. 95% O. Find the empirical formula. � When percentages are given, use the values with grams as the unit. 1. Find the number of moles of each element. Conversion factor: molar mass mol S = 40. 05 g S 1 mol S 32. 1 g S = 1. 25 mol S mol O = 59. 95 g O 1 mol O 16. 0 g O = 3. 75 mol O

DETERMINING EMPIRICAL FORMULA � Recall that the subscripts in a chemical formula indicate the number of moles of each element � If the compound contains sulfur and oxygen in the ratio of 1. 25 to 3. 75, then the formula could be written as S 1. 25 O 3. 75 � What’s the problem here?

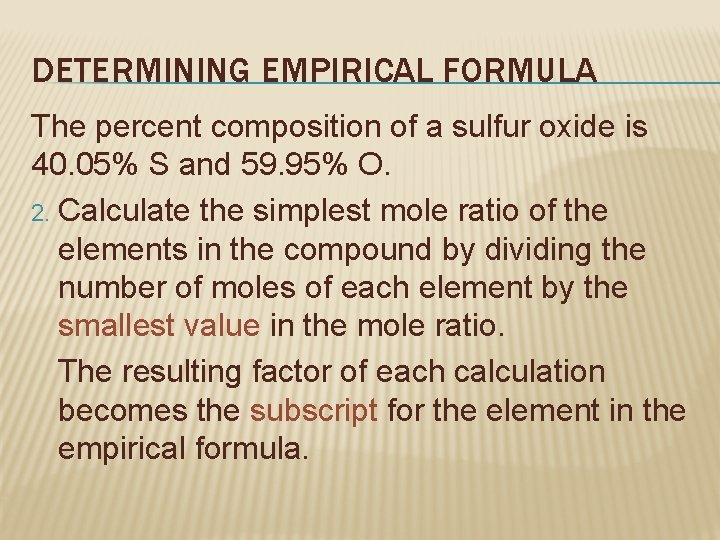
DETERMINING EMPIRICAL FORMULA The percent composition of a sulfur oxide is 40. 05% S and 59. 95% O. 2. Calculate the simplest mole ratio of the elements in the compound by dividing the number of moles of each element by the smallest value in the mole ratio. The resulting factor of each calculation becomes the subscript for the element in the empirical formula.

DETERMINING EMPIRICAL FORMULA The percent composition of a sulfur oxide is 40. 05% S and 59. 95% O. 2. Ratio of S to O is 1. 25 : 3. 75 1. 25 Smallest value: 1. 25 or 3. 75? Subscript for S: 1. 25 mol =1 1. 25 mol Subscript for O: 3. 75 mol =3 1. 25 mol If results are not whole numbers, must multiply both by a factor that results in whole numbers.

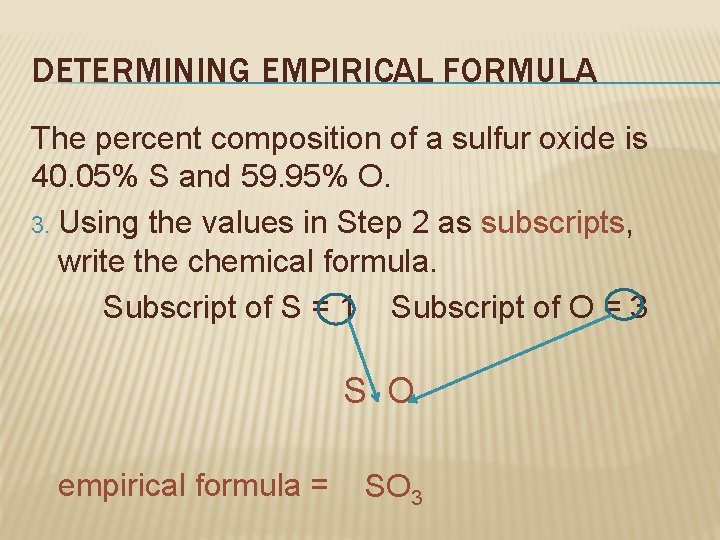
DETERMINING EMPIRICAL FORMULA The percent composition of a sulfur oxide is 40. 05% S and 59. 95% O. 3. Using the values in Step 2 as subscripts, write the chemical formula. Subscript of S = 1 Subscript of O = 3 S O empirical formula = SO 3

MOLECULAR FORMULA

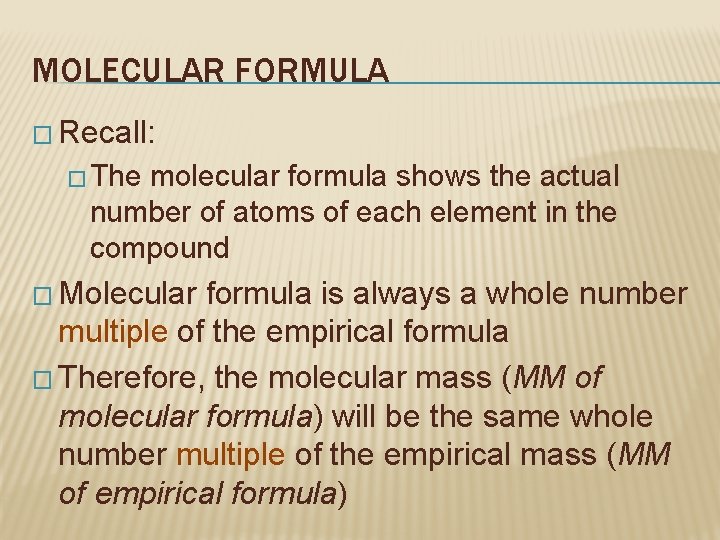
MOLECULAR FORMULA � Recall: � The molecular formula shows the actual number of atoms of each element in the compound � Molecular formula is always a whole number multiple of the empirical formula � Therefore, the molecular mass (MM of molecular formula) will be the same whole number multiple of the empirical mass (MM of empirical formula)

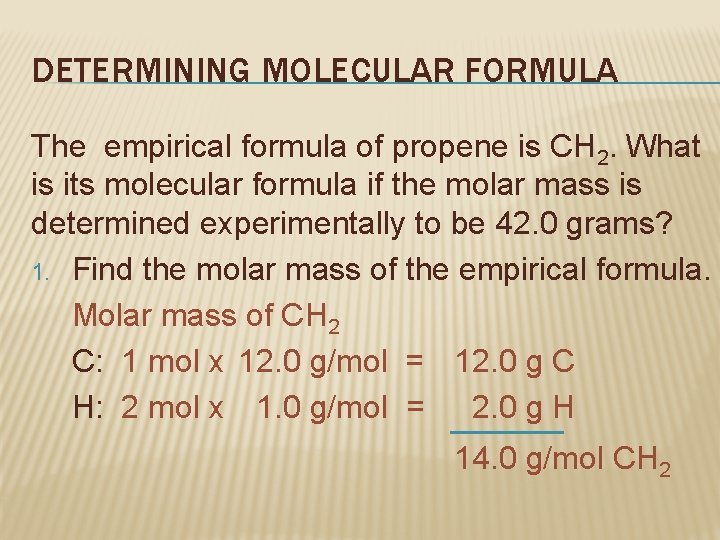
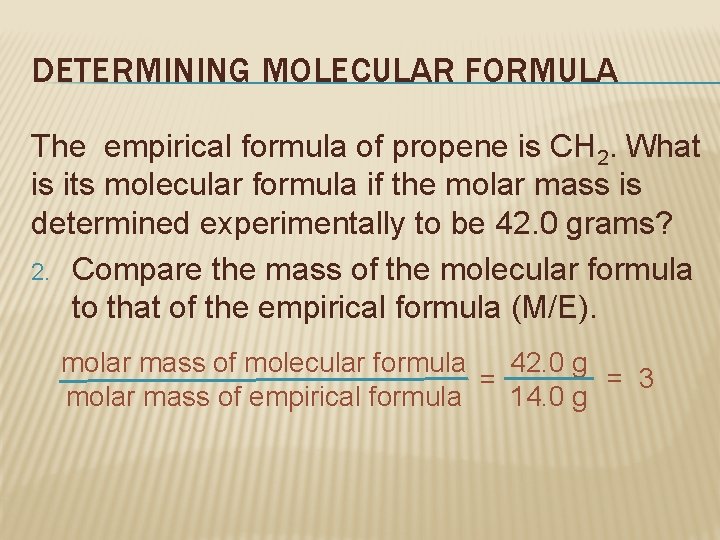
DETERMINING MOLECULAR FORMULA The empirical formula of propene is CH 2. What is its molecular formula if the molar mass is determined experimentally to be 42. 0 grams? 1. Find the molar mass of the empirical formula. Molar mass of CH 2 C: 1 mol x 12. 0 g/mol = 12. 0 g C H: 2 mol x 1. 0 g/mol = 2. 0 g H 14. 0 g/mol CH 2

DETERMINING MOLECULAR FORMULA The empirical formula of propene is CH 2. What is its molecular formula if the molar mass is determined experimentally to be 42. 0 grams? 2. Compare the mass of the molecular formula to that of the empirical formula (M/E). 42. 0 g molar mass of molecular formula = 3 = 14. 0 g molar mass of empirical formula

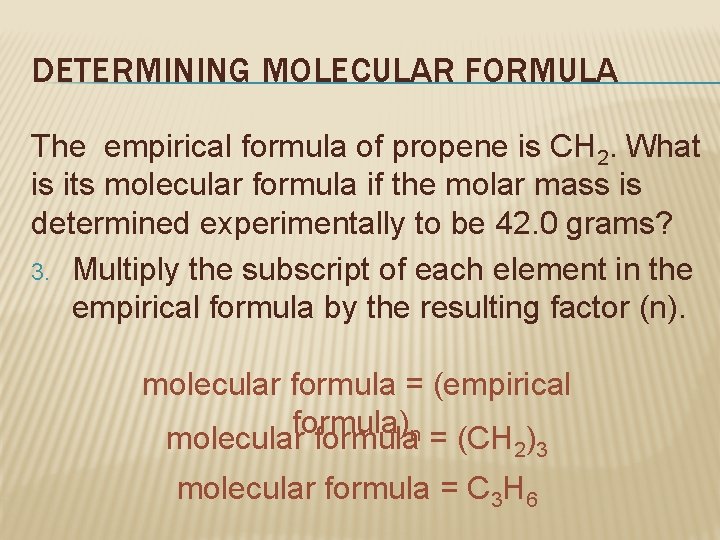
DETERMINING MOLECULAR FORMULA The empirical formula of propene is CH 2. What is its molecular formula if the molar mass is determined experimentally to be 42. 0 grams? 3. Multiply the subscript of each element in the empirical formula by the resulting factor (n). molecular formula = (empirical formula) molecular formulan = (CH ) 2 3 molecular formula = C 3 H 6