UNIT 7 TEST Multiple choice answers with explanation



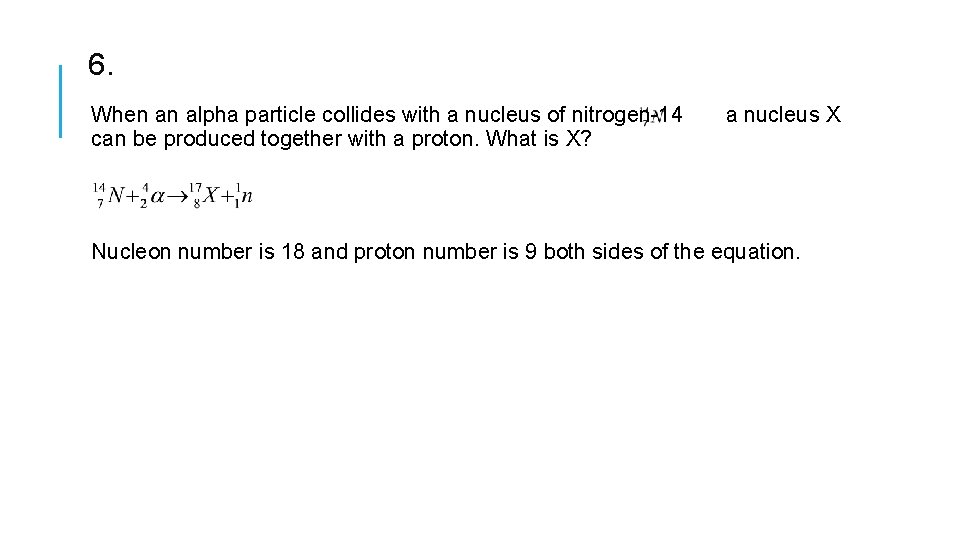


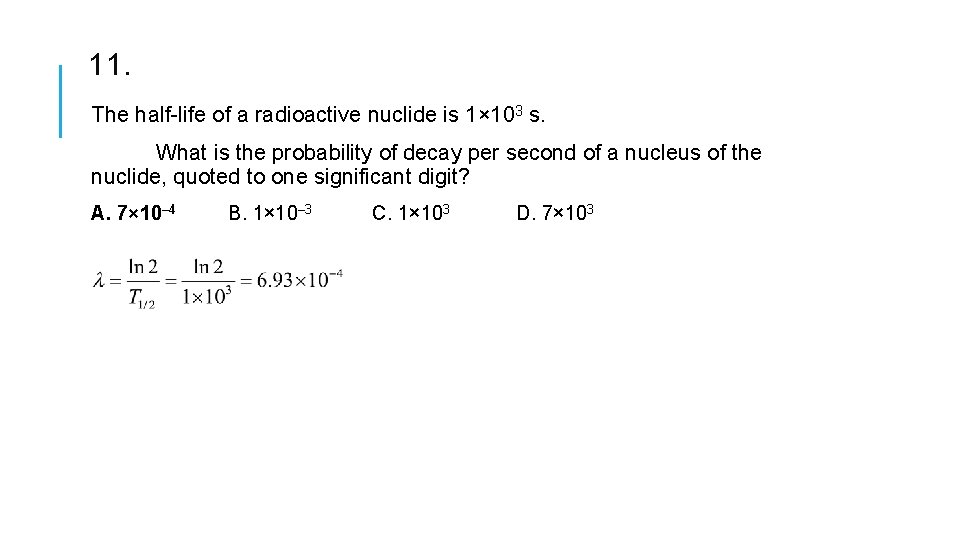

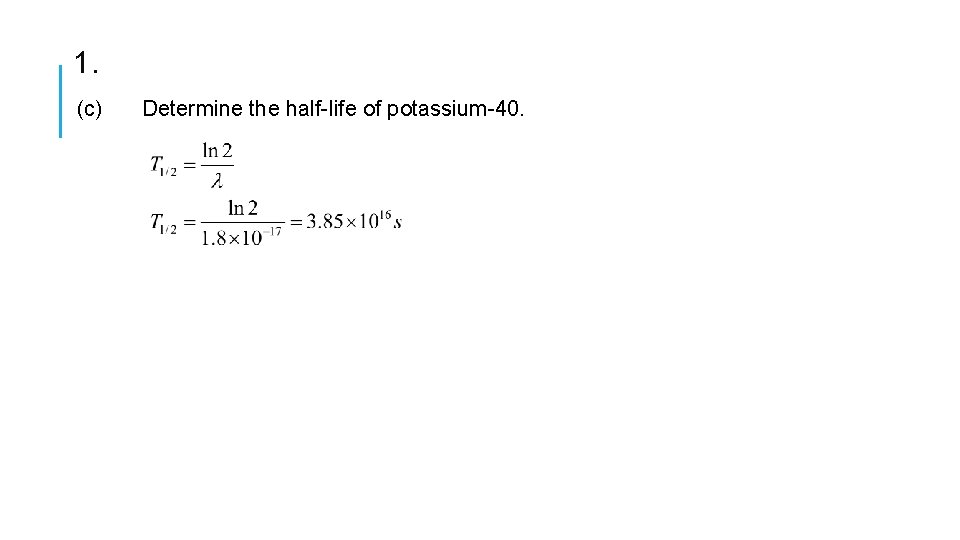





- Slides: 25

UNIT 7 TEST Multiple choice answers with explanation.

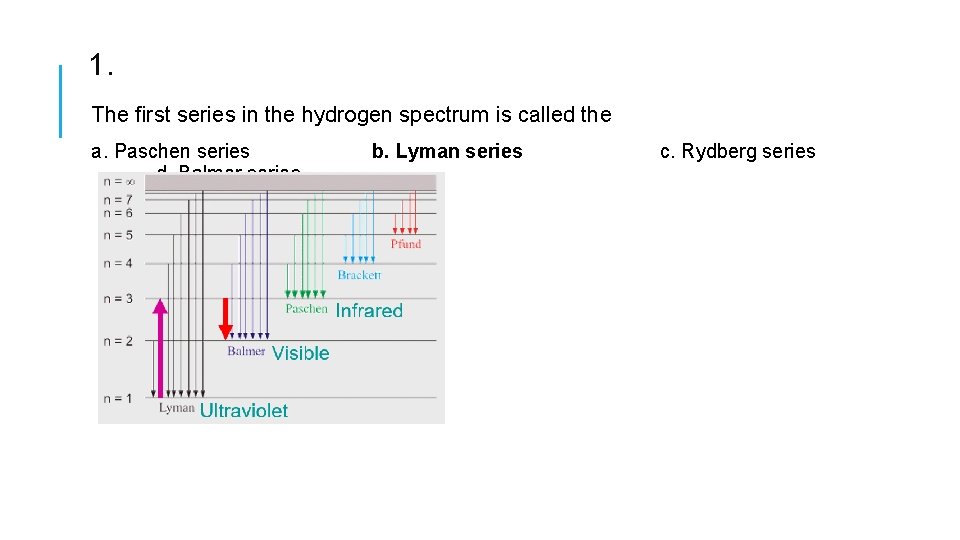
1. The first series in the hydrogen spectrum is called the a. Paschen series d. Balmer series b. Lyman series c. Rydberg series

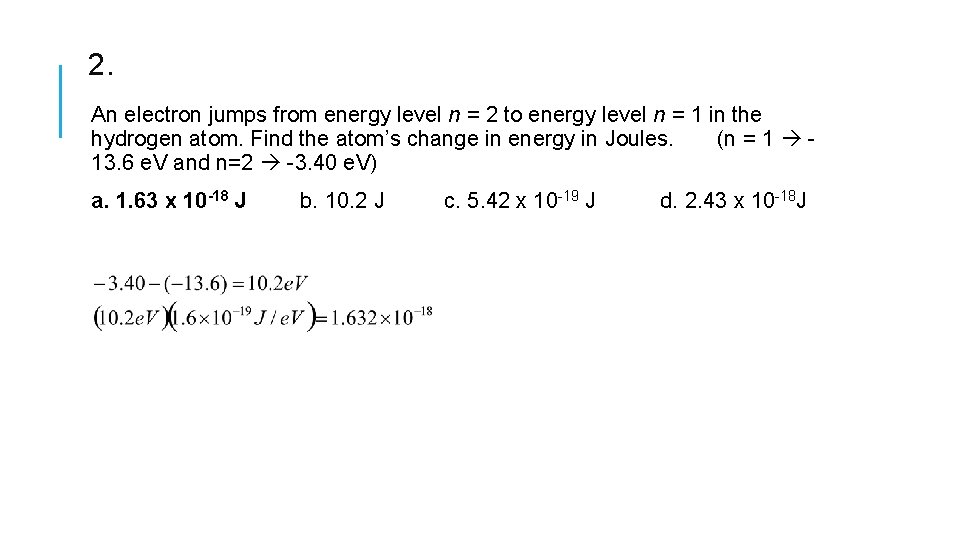
2. An electron jumps from energy level n = 2 to energy level n = 1 in the hydrogen atom. Find the atom’s change in energy in Joules. (n = 1 13. 6 e. V and n=2 -3. 40 e. V) a. 1. 63 x 10 -18 J b. 10. 2 J c. 5. 42 x 10 -19 J d. 2. 43 x 10 -18 J

3. When electrons of suitable energy travel through a thin layer of graphite, a pattern of concentric circles is produced on a screen. The production of this pattern is evidence for A. the wave nature of the electron. B. the nuclear model of the atom. C. the particle nature of the electron. D. the existence of X-rays.

4. The nucleus undergoes radioactive decay to the nucleus The particles emitted in the decay are A. a positron and an antineutrino. C. a positron and a neutrino. B. an electron and an antineutrino. D. an electron and a neutrino. Proton becomes a neutron losing positive charge in the form of a positron. Neutrino is paired with a positron emission.

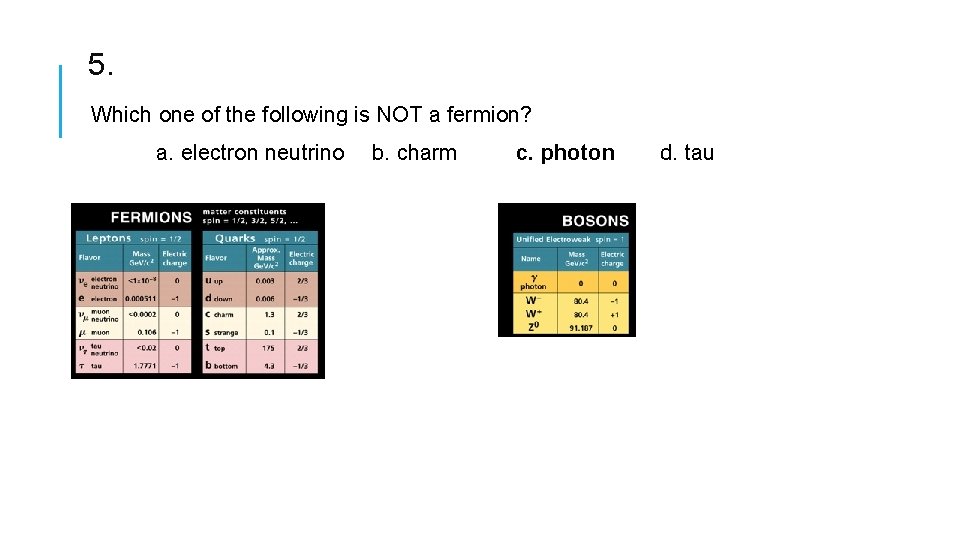
5. Which one of the following is NOT a fermion? a. electron neutrino b. charm c. photon d. tau
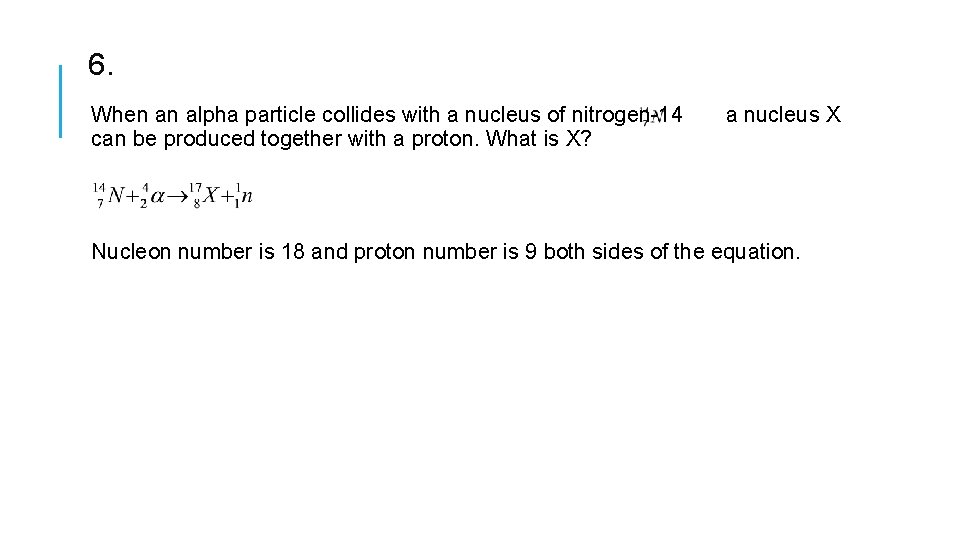
6. When an alpha particle collides with a nucleus of nitrogen-14 a nucleus X can be produced together with a proton. What is X? Nucleon number is 18 and proton number is 9 both sides of the equation.

7. The mass defect for deuterium is 4 x 10– 30 kg. What is the binding energy of deuterium? a. 4 x 10– 7 e. V b. 8 x 10– 2 e. V c. 2 x 106 e. V d. 2 x 1012 e. V

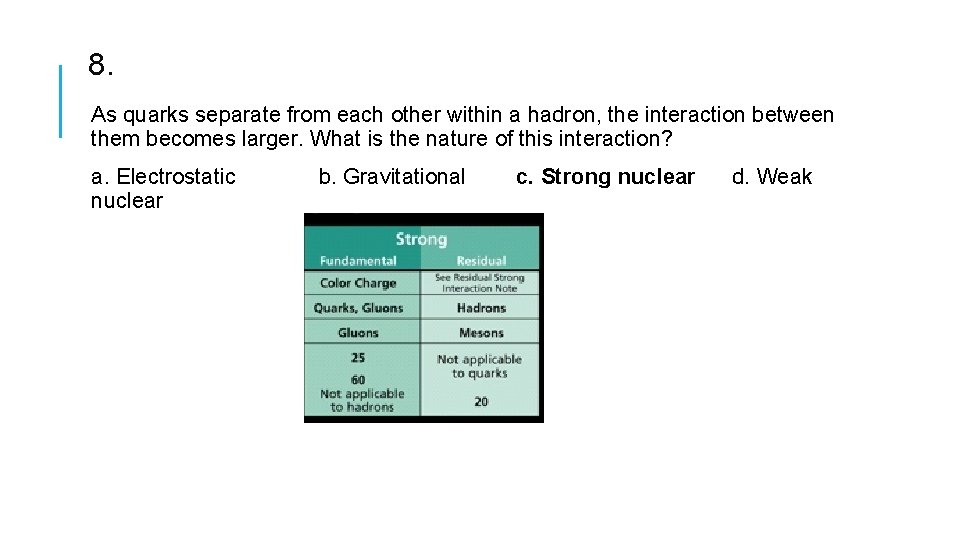
8. As quarks separate from each other within a hadron, the interaction between them becomes larger. What is the nature of this interaction? a. Electrostatic nuclear b. Gravitational c. Strong nuclear d. Weak

9. Photons of energy 2. 3 e. V are incident on a low-pressure vapour. The energy levels of the atoms in the vapour are shown. What energy transition will occur when a photon is absorbed by the vapour? a. – 3. 9 e. V to – 1. 6 e. V b. – 1. 6 e. V to 0 e. V c. – 1. 6 e. V to – 3. 9 e. V d. 0 e. V to – 1. 6 e. V Absorption low energy level to higher level

10. Which of the following correctly identifies the mass and momentum of a photon? Mass Momentum A. B. C. D. zero non-zero Although the photon has zero rest mass, it does have energy. (E = mc 2) p = E/c
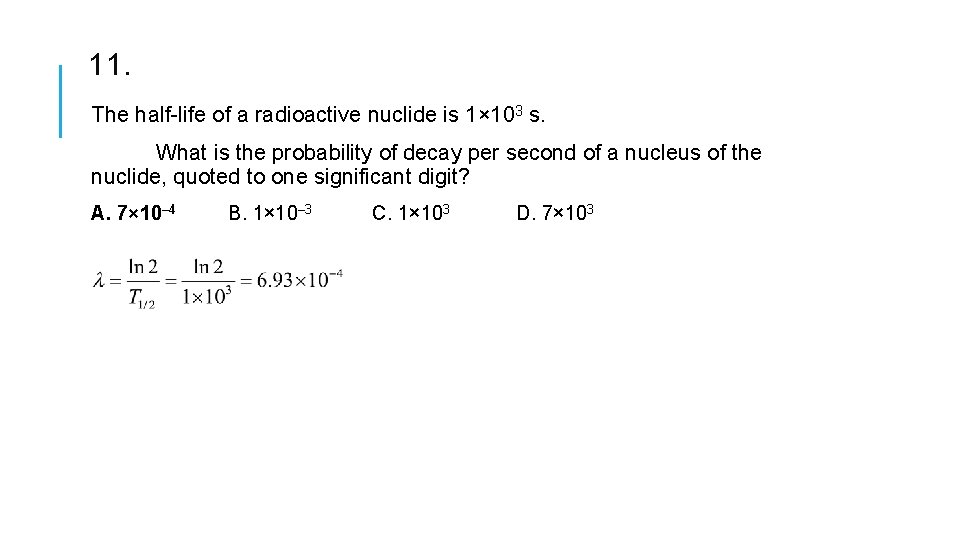
11. The half-life of a radioactive nuclide is 1× 103 s. What is the probability of decay per second of a nucleus of the nuclide, quoted to one significant digit? A. 7× 10– 4 B. 1× 10– 3 C. 1× 103 D. 7× 103

12. One possible fission reaction can be represented by the equation The extra EU, ETe and EZr are the binding energies of uranium, tellurium and zirconium respectively. Binding energy is defined as a positive quantity. It may be deduced that A. EU = ETe + EZr. B. EU > ETe + EZr. C. EU < ETe + EZr. D. EU = ETe − EZr.

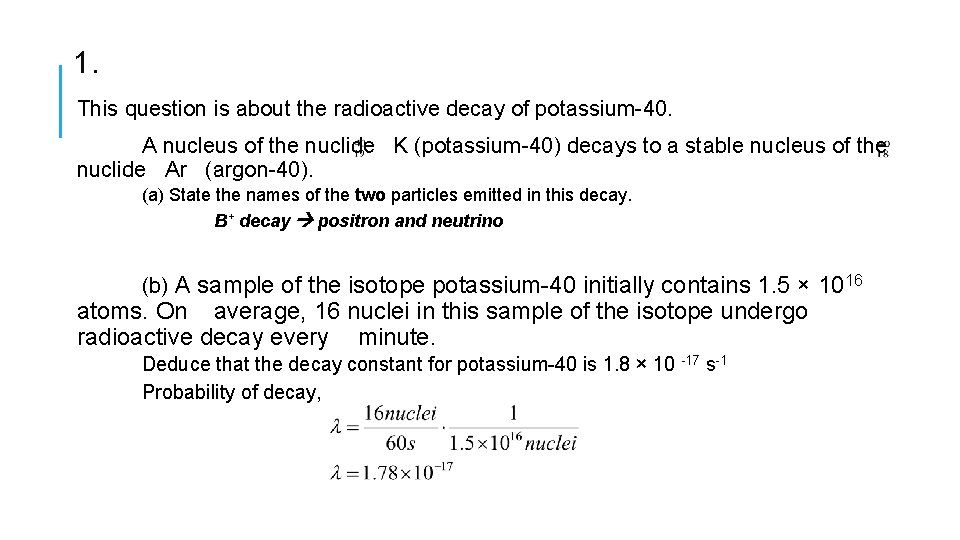
1. This question is about the radioactive decay of potassium-40. A nucleus of the nuclide K (potassium-40) decays to a stable nucleus of the nuclide Ar (argon-40). (a) State the names of the two particles emitted in this decay. B+ decay positron and neutrino (b) A sample of the isotope potassium-40 initially contains 1. 5 × 1016 atoms. On average, 16 nuclei in this sample of the isotope undergo radioactive decay every minute. Deduce that the decay constant for potassium-40 is 1. 8 × 10 -17 s-1 Probability of decay,
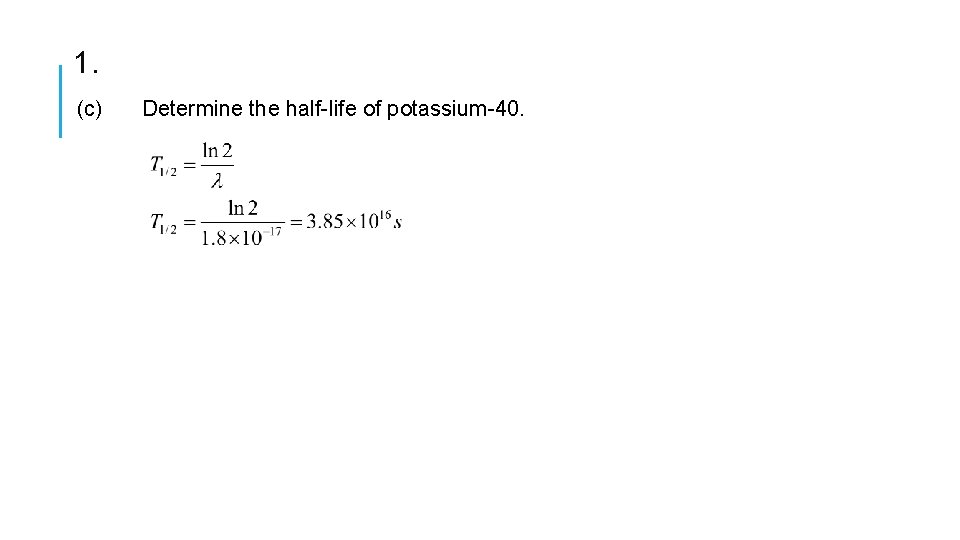
1. (c) Determine the half-life of potassium-40.

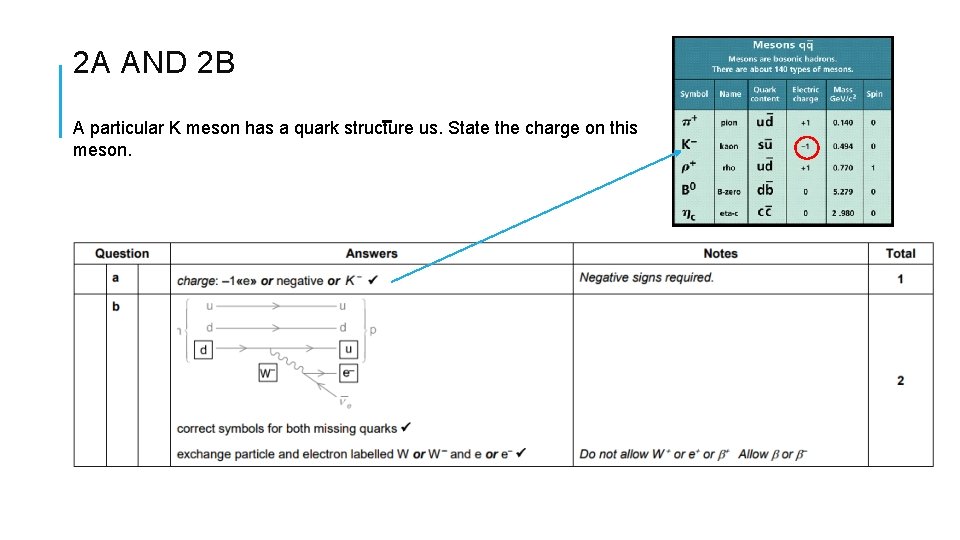
2 A AND 2 B A particular K meson has a quark structure us. State the charge on this meson.

2 C. decay products include an electron that has mass OR products have energy that has a mass equivalent OR mass/mass defect/binding energy converted to mass/energy of decay products mass C-14 > mass N-14 OR mass of n > mass of p OR mass of d > mass of u

3. This question is about nuclear decay. (a) (i) Describe the phenomenon of natural radioactive decay. emission of particles and/or e. m. radiation from unstable nucleus; not affected by temperature/environment / is spontaneous process; constant probability of decay (per unit time) / is random process; activity/number of unstable nuclei in sample reduces exponentially; daughter nucleus is (energetically) more stable;

3. (ii) Ionizing radiation is emitted during radioactive decay. Explain what is meant by the term ionizing. electron(s) ejected from (neutral) atoms; to form positively and negatively charged particles; (do not allow “ions”)

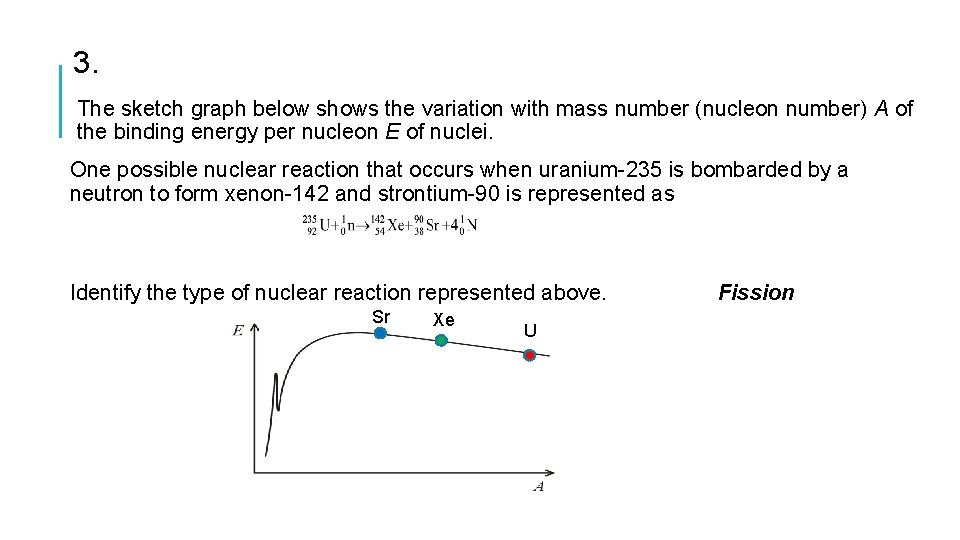
3. The sketch graph below shows the variation with mass number (nucleon number) A of the binding energy per nucleon E of nuclei. One possible nuclear reaction that occurs when uranium-235 is bombarded by a neutron to form xenon-142 and strontium-90 is represented as Identify the type of nuclear reaction represented above. Sr Xe U Fission

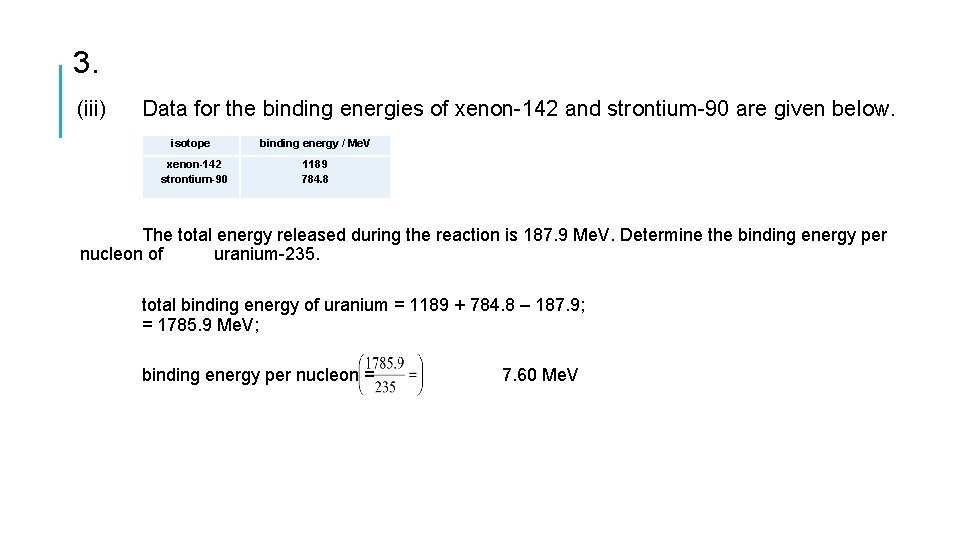
3. (iii) Data for the binding energies of xenon-142 and strontium-90 are given below. isotope xenon-142 strontium-90 binding energy / Me. V 1189 784. 8 The total energy released during the reaction is 187. 9 Me. V. Determine the binding energy per nucleon of uranium-235. total binding energy of uranium = 1189 + 784. 8 – 187. 9; = 1785. 9 Me. V; binding energy per nucleon = 7. 60 Me. V

3. (iv) State why binding energy of the neutrons formed in the reaction is not quoted. binding energy is zero because neutrons are separate particles;

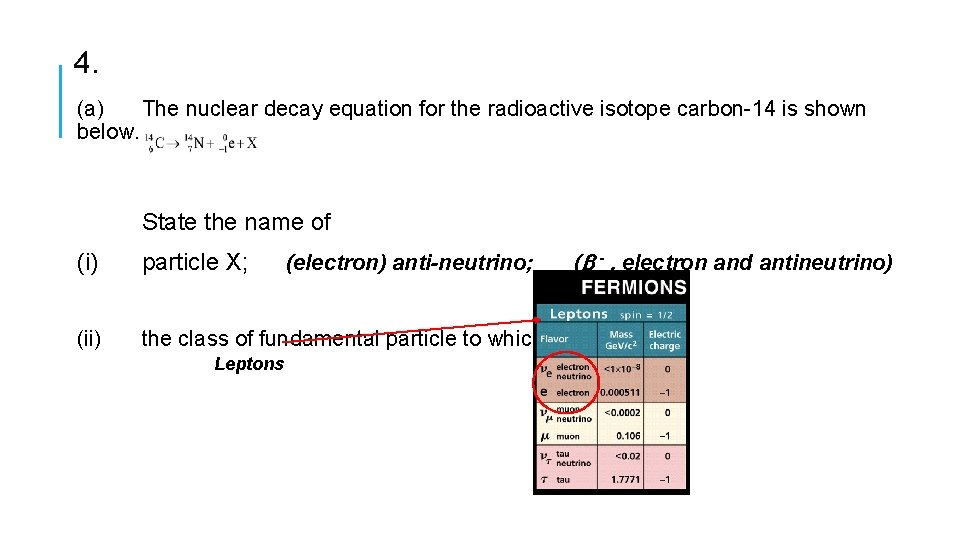
4. (a) The nuclear decay equation for the radioactive isotope carbon-14 is shown below. State the name of (i) particle X; (ii) the class of fundamental particle to which Leptons (electron) anti-neutrino; (b - , electron and antineutrino)

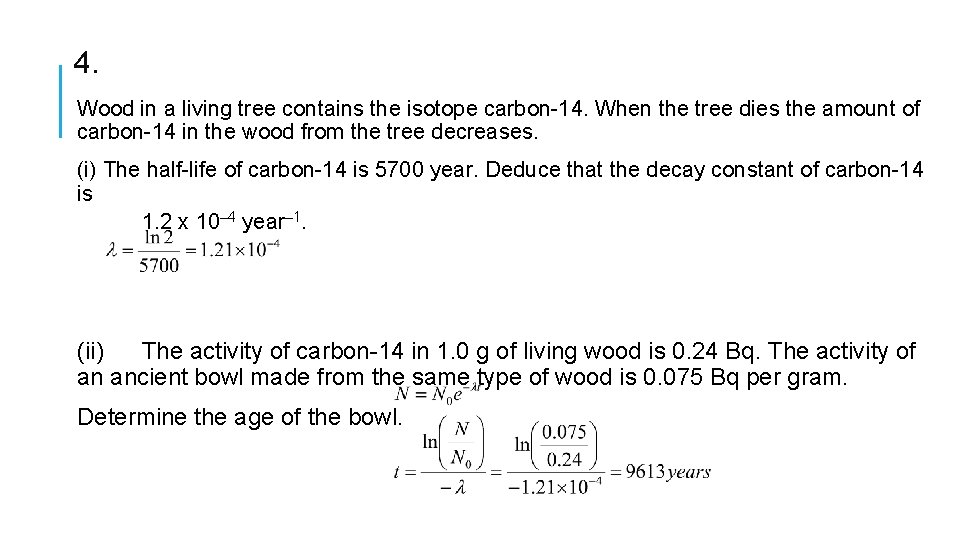
4. Wood in a living tree contains the isotope carbon-14. When the tree dies the amount of carbon-14 in the wood from the tree decreases. (i) The half-life of carbon-14 is 5700 year. Deduce that the decay constant of carbon-14 is 1. 2 x 10– 4 year– 1. (ii) The activity of carbon-14 in 1. 0 g of living wood is 0. 24 Bq. The activity of an ancient bowl made from the same type of wood is 0. 075 Bq per gram. Determine the age of the bowl.

4. (c) Outline how the half-life of carbon-14 may be determined experimentally. measure activity of source; determine number of molecules chemically; activity = l x N , hence half-life;