Unit 7 Solution Chemistry Section 2 Percent Composition









- Slides: 10

Unit 7: Solution Chemistry Section 2: Percent Composition and Colligative Properties

Percent Solutions • Used by consumers to determine the concentration of a solution – Chemists prefer to use molarity and molality • There are three formulas to calculate percent solution – Consumer products don’t indicate which formula they are using – Each manufacturer chooses their own method that they feel is most suitable for their product

Percent Solutions • Weight/weight solutions: – Ex: Calculate the weight/weight percent solution formed by mixing 5 grams of solute with 95 grams of solvent. solution

Percent Solutions • Weight/volume solutions: – Ex: Calculate the weight/volume percent solution formed by mixing 2 grams of solute with enough water to make 50 m. L of solution.

Percent Solutions • Volume/volume solutions: – Ex: Calculate the volume/volume percent solution formed by mixing 5 m. L of alcohol with enough water to make 100 m. L of solution.

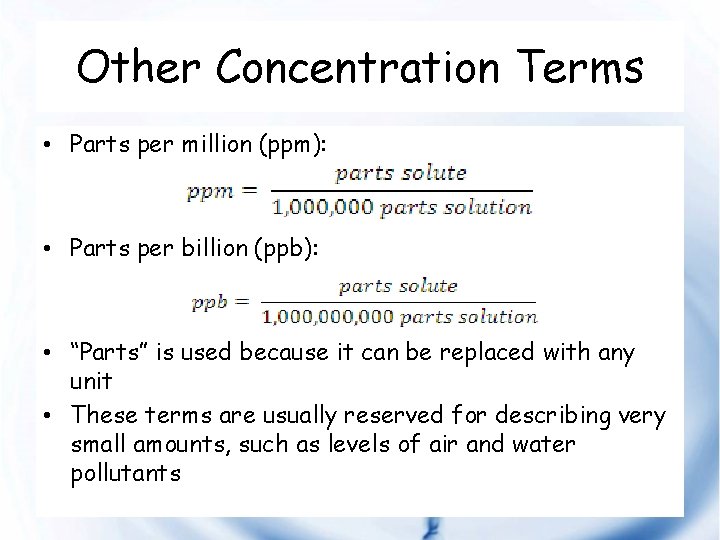
Other Concentration Terms • Parts per million (ppm): • Parts per billion (ppb): • “Parts” is used because it can be replaced with any unit • These terms are usually reserved for describing very small amounts, such as levels of air and water pollutants

Colligative Properties • Properties of solutions that depend on the number of molecules in a given volume of solvent – Vapor pressure, boiling point, and freezing point • They do not depend on the properties of the molecules – size or mass

Vapor Pressure • Caused by the evaporation of molecules at the surface of a liquid – The escaping molecules exert an upward pressure as they leave the liquid • Vapor pressure of a liquid is reduced by the presence of solute particles in the liquid b/c the solute molecules reduce the number of liquid molecules on the surface – Vapor pressure of water is greater than the vapor pressure of a sugar solution b/c the presence of the sugar molecules at the liquid’s surface decreases the # of water molecules that are free to evaporate – Gasoline has a greater vapor pressure than syrup b/c gasoline molecules evaporate more readily than syrup molecules

Boiling Point • Related to vapor pressure • Is the temperature at which the vapor pressure escaping from a liquid is equal to the atmospheric pressure pushing down on the surface of the liquid – The boiling point of a liquid increases when molecules of a solute are added

Freezing Point • When a liquid freezes, its molecules slow to the point that they no longer slide past each other – The freezing point of a liquid must be lowered even more when molecules of a solute are added