Unit 7 Periodic Table Chapters 6 7 Chemistry

Unit 7: Periodic Table Chapters 6 & 7 Chemistry 1 L Cypress Creek High School

Part 4: Periodic Trends
Periodic Table Trends v. Patterns on the periodic table v. Atomic Radius v. Ionic Radius v. Electronegativity v. Ionization Energy

Periodic Trends v. Depend upon 4 important factors… v. Energy levels – the horizontal rows; ranked from 1 -7 based on energy and distance from the nucleus v. Valence electrons – number of electrons in outermost energy level v. Shielding effect – the decrease in the attraction between the outer electrons and the nucleus due to the presence of other electrons between them v. Nuclear charge – depends on the number of protons – the more protons in the nucleus, the greater pull they have on their surrounding electrons
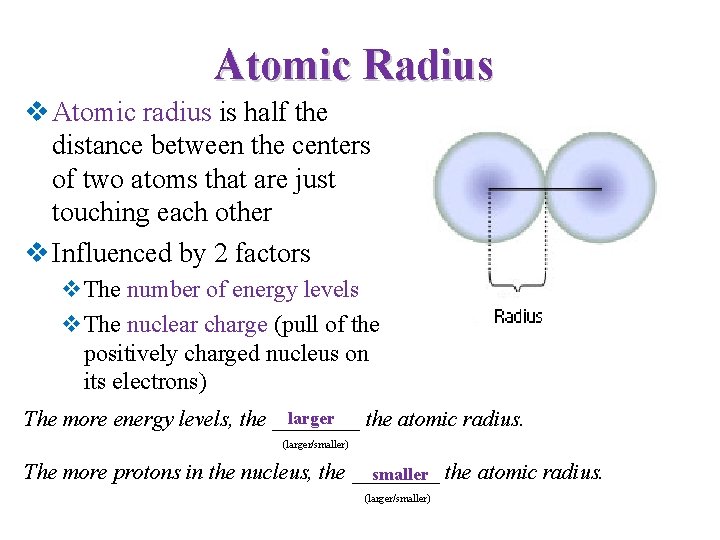
Atomic Radius v Atomic radius is half the distance between the centers of two atoms that are just touching each other v Influenced by 2 factors v. The number of energy levels v. The nuclear charge (pull of the positively charged nucleus on its electrons) larger The more energy levels, the ____ the atomic radius. (larger/smaller) The more protons in the nucleus, the ____ smaller the atomic radius. (larger/smaller)
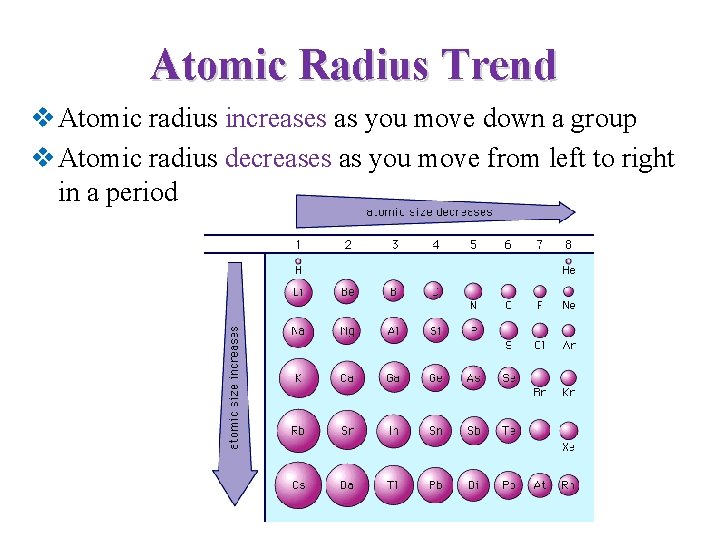
Atomic Radius Trend v Atomic radius increases as you move down a group v Atomic radius decreases as you move from left to right in a period
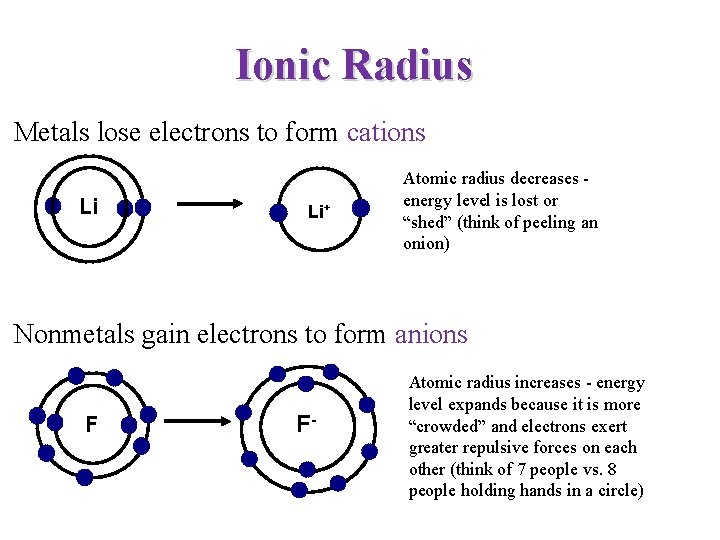
Ionic Radius Metals lose electrons to form cations Li Li+ Atomic radius decreases energy level is lost or “shed” (think of peeling an onion) Nonmetals gain electrons to form anions F F- Atomic radius increases - energy level expands because it is more “crowded” and electrons exert greater repulsive forces on each other (think of 7 people vs. 8 people holding hands in a circle)
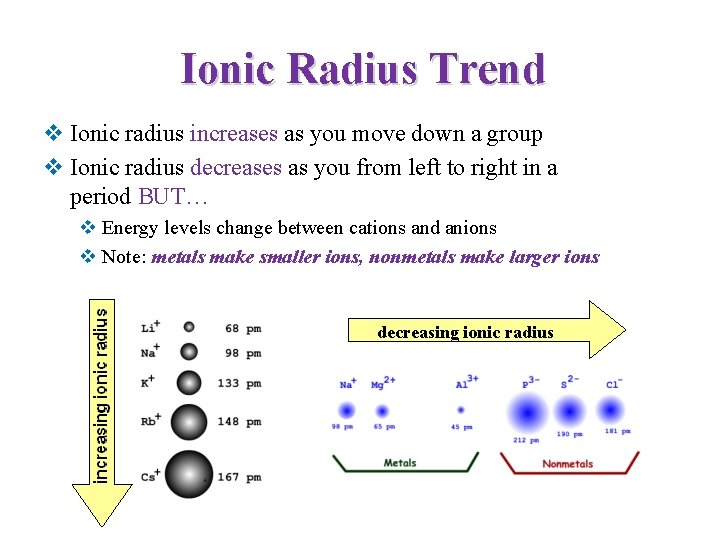
Ionic Radius Trend v Ionic radius increases as you move down a group v Ionic radius decreases as you from left to right in a period BUT… v Energy levels change between cations and anions v Note: metals make smaller ions, nonmetals make larger ions decreasing ionic radius
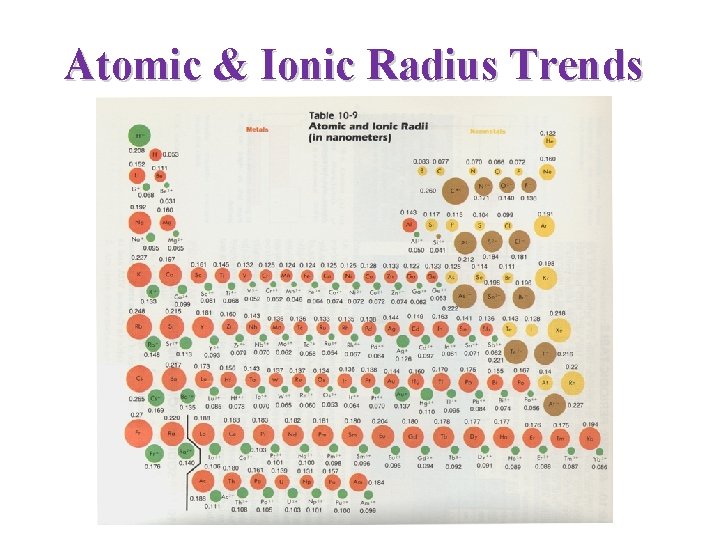
Atomic & Ionic Radius Trends
Electronegativity v Electronegativity is a measure of how easily an atom attracts the valence electrons of another atom v Numbers are assigned to each element to rate the electronegativity (from 0. 7 to 4. 0) v Low electronegativity = does not want to attract valence electrons (metals) v High electronegativity = really wants to attract valence electrons (nonmetals) v Influenced by 2 factors: v Valence electrons v Shielding
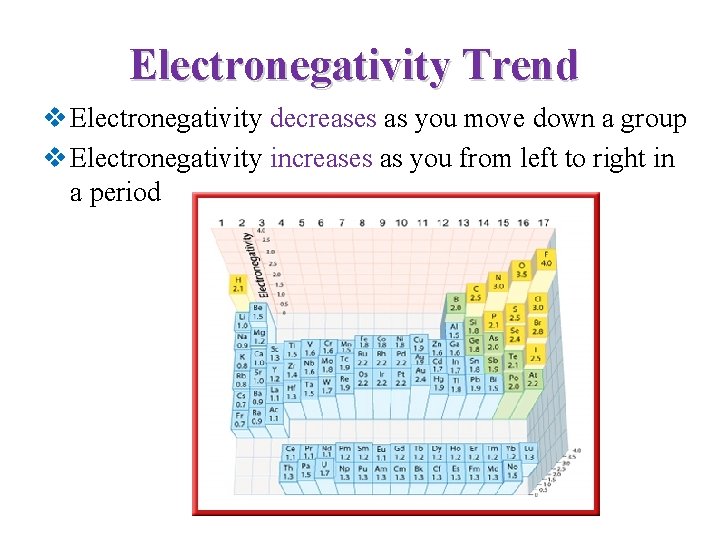
Electronegativity Trend v Electronegativity decreases as you move down a group v Electronegativity increases as you from left to right in a period
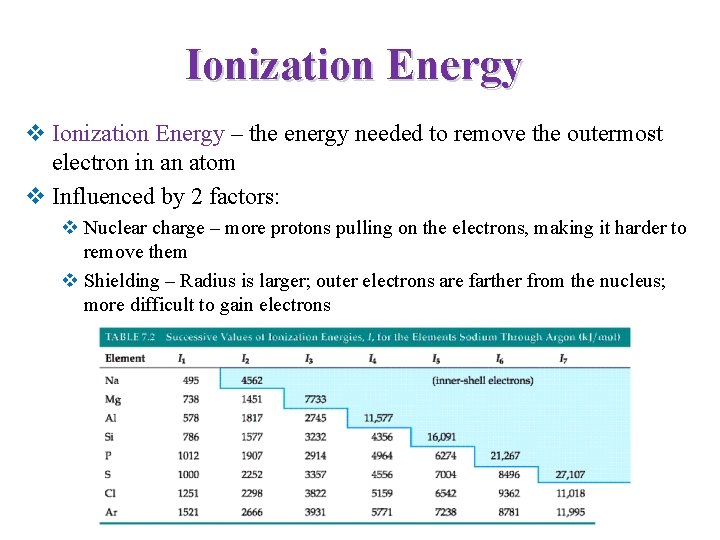
Ionization Energy v Ionization Energy – the energy needed to remove the outermost electron in an atom v Influenced by 2 factors: v Nuclear charge – more protons pulling on the electrons, making it harder to remove them v Shielding – Radius is larger; outer electrons are farther from the nucleus; more difficult to gain electrons
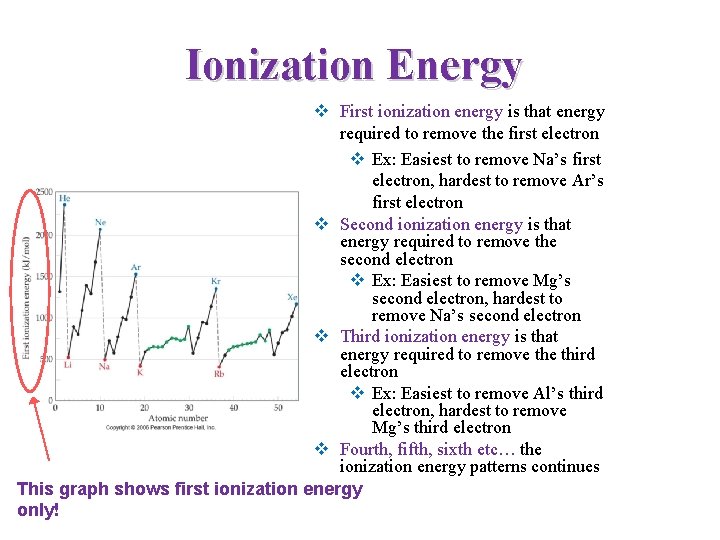
Ionization Energy v First ionization energy is that energy required to remove the first electron v Ex: Easiest to remove Na’s first electron, hardest to remove Ar’s first electron v Second ionization energy is that energy required to remove the second electron v Ex: Easiest to remove Mg’s second electron, hardest to remove Na’s second electron v Third ionization energy is that energy required to remove third electron v Ex: Easiest to remove Al’s third electron, hardest to remove Mg’s third electron v Fourth, fifth, sixth etc… the ionization energy patterns continues This graph shows first ionization energy only!
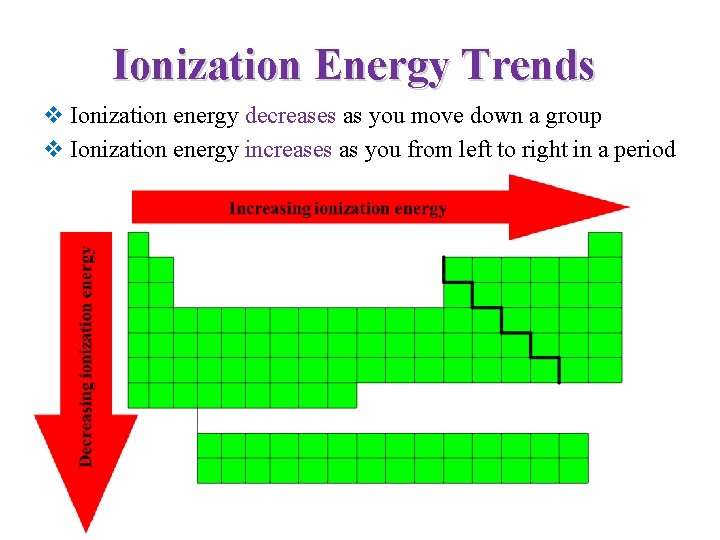
Ionization Energy Trends v Ionization energy decreases as you move down a group v Ionization energy increases as you from left to right in a period
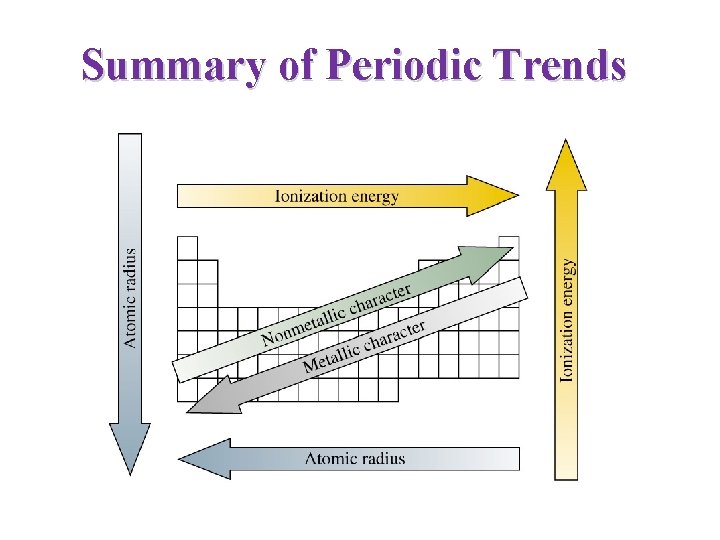
Summary of Periodic Trends
- Slides: 15