UNIT 7 MOLECULES TO METABOLISM Essential Idea Living
UNIT 7: MOLECULES TO METABOLISM Essential Idea: Living organisms control their composition by a complex web of chemical reactions. IUBMB-Sigma-Nicholson Metabolic Pathways Chart
2. 1. U 1 MOLECULAR BIOLOGY EXPLAINS LIVING PROCESSES IN TERMS OF THE CHEMICAL SUBSTANCES INVOLVED. What is molecular biology? • The study of the structure and function of biologically important molecules. • These molecules are classified into 4 groups and water. • Nucleic Acids • Proteins • Lipids • Carbohydrates • Each of these molecules has a specific structure and function and work together to ensure the needs of the cell are met.

ORGANIC VERSUS INORGANIC • Organic molecules refers to molecules that contain a carbon skeleton • Inorganic refers to carbon dioxide and all molecules without carbon

2. 1. U 2 CARBON ATOMS CAN FORM FOUR COVALENT BONDS ALLOWING A DIVERSITY OF STABLE COMPOUNDS TO EXIST.
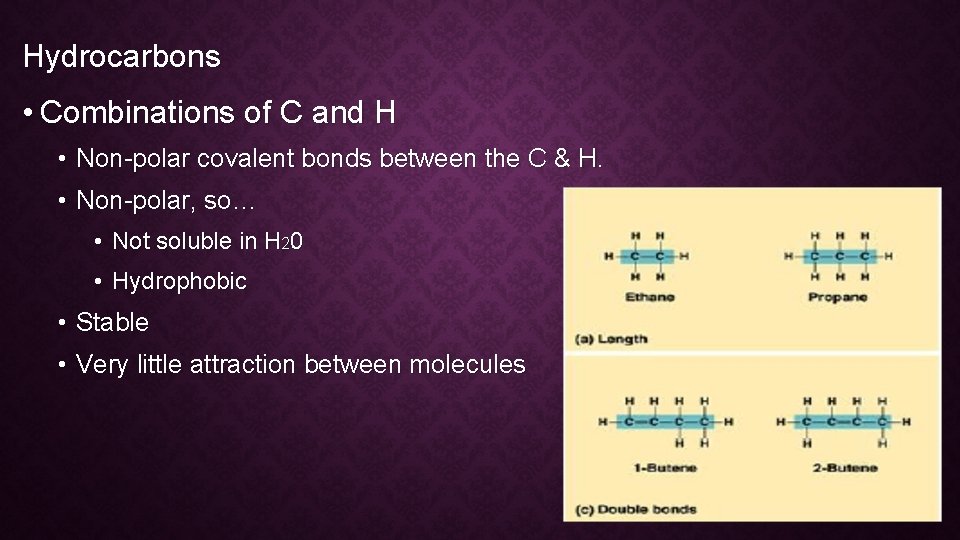
Hydrocarbons • Combinations of C and H • Non-polar covalent bonds between the C & H. • Non-polar, so… • Not soluble in H 20 • Hydrophobic • Stable • Very little attraction between molecules
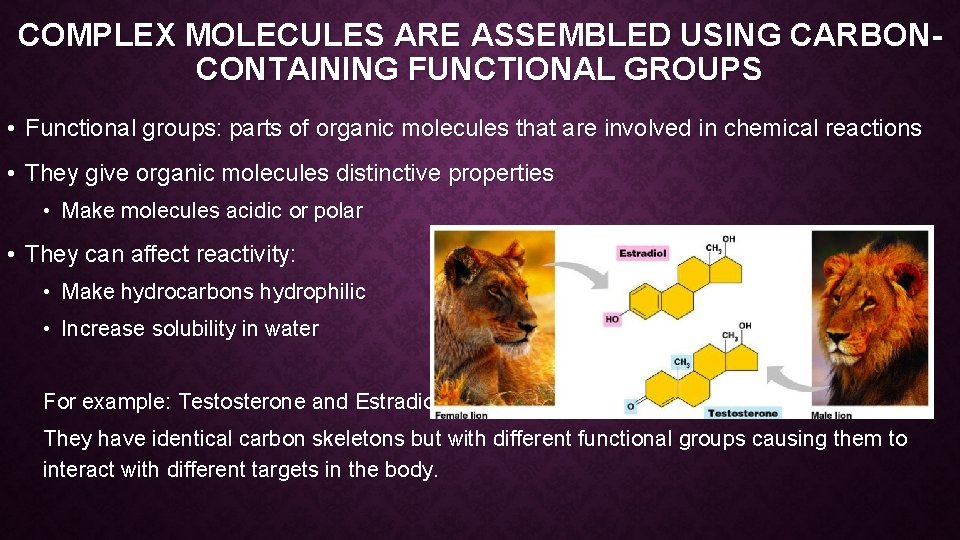
COMPLEX MOLECULES ARE ASSEMBLED USING CARBONCONTAINING FUNCTIONAL GROUPS • Functional groups: parts of organic molecules that are involved in chemical reactions • They give organic molecules distinctive properties • Make molecules acidic or polar • They can affect reactivity: • Make hydrocarbons hydrophilic • Increase solubility in water For example: Testosterone and Estradiol They have identical carbon skeletons but with different functional groups causing them to interact with different targets in the body.

HYDROXYL Polar Molecules!
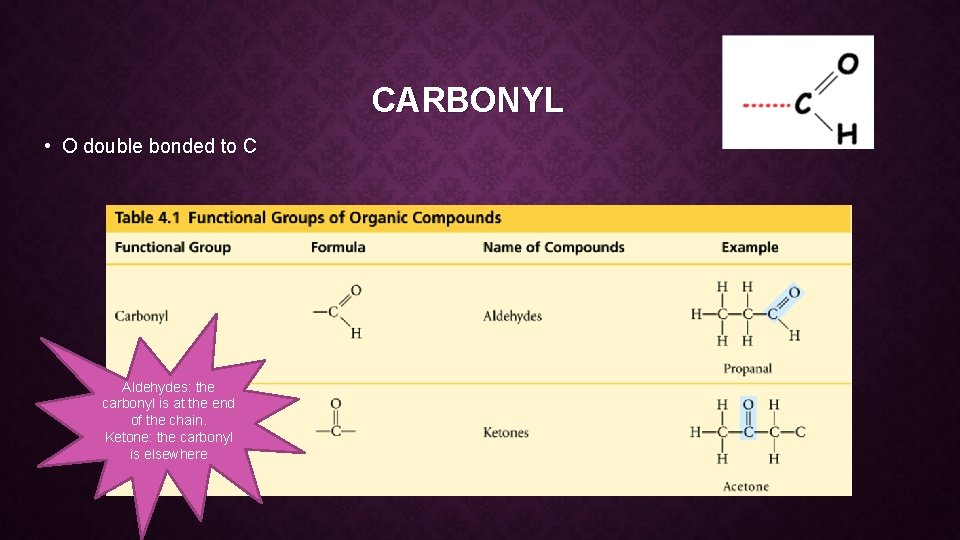
CARBONYL • O double bonded to C Aldehydes: the carbonyl is at the end of the chain. Ketone: the carbonyl is elsewhere
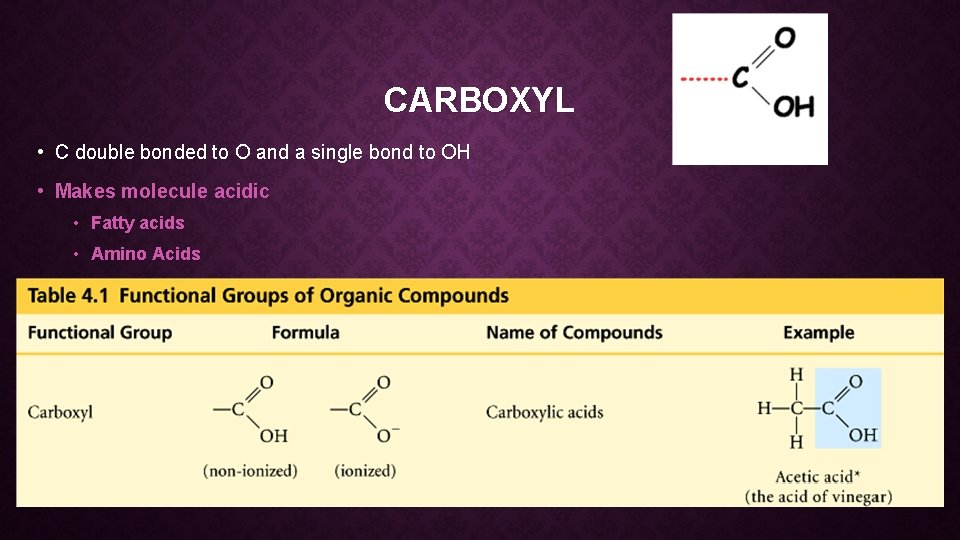
CARBOXYL • C double bonded to O and a single bond to OH • Makes molecule acidic • Fatty acids • Amino Acids
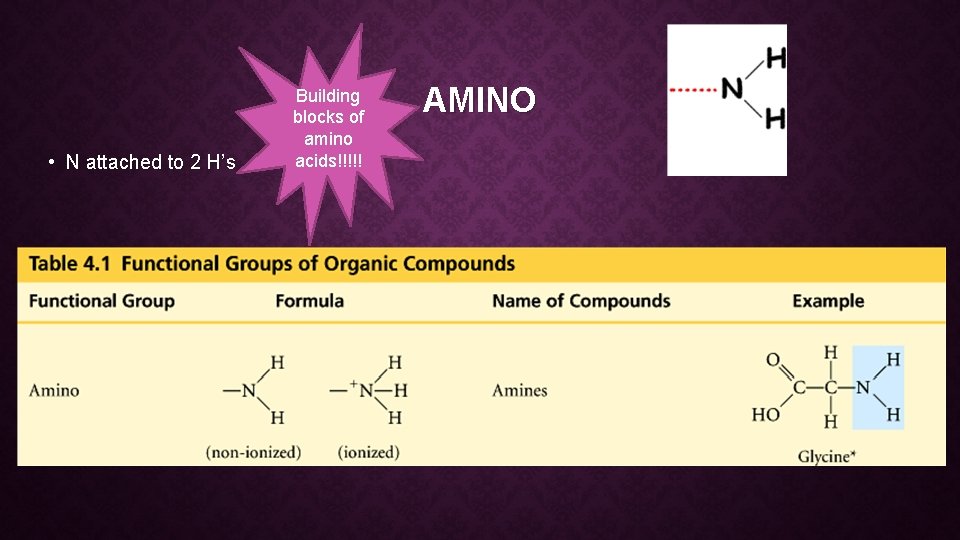
• N attached to 2 H’s Building blocks of amino acids!!!!! AMINO
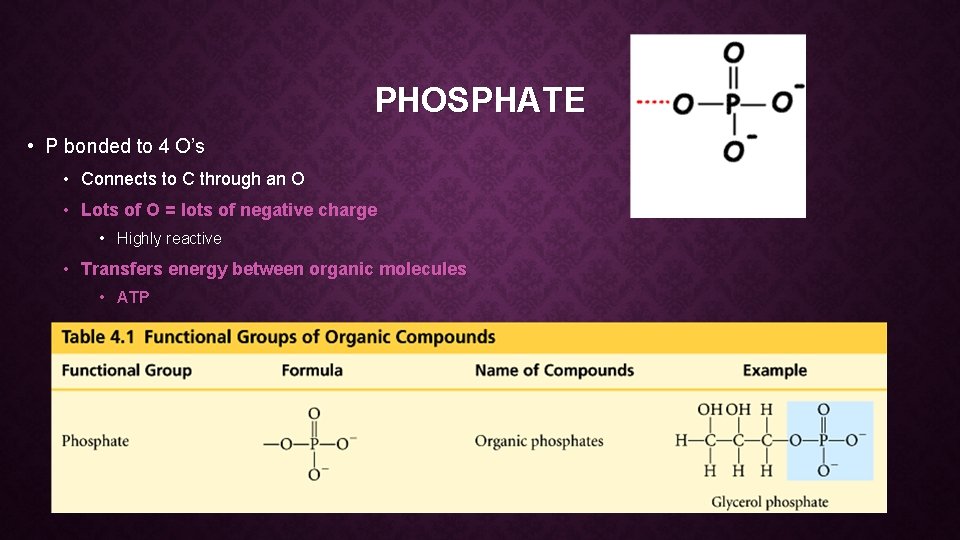
PHOSPHATE • P bonded to 4 O’s • Connects to C through an O • Lots of O = lots of negative charge • Highly reactive • Transfers energy between organic molecules • ATP
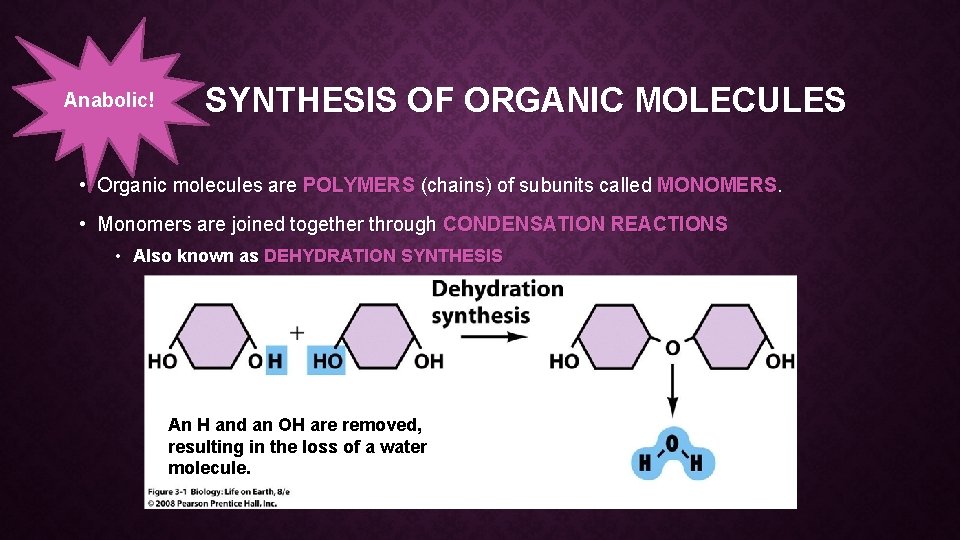
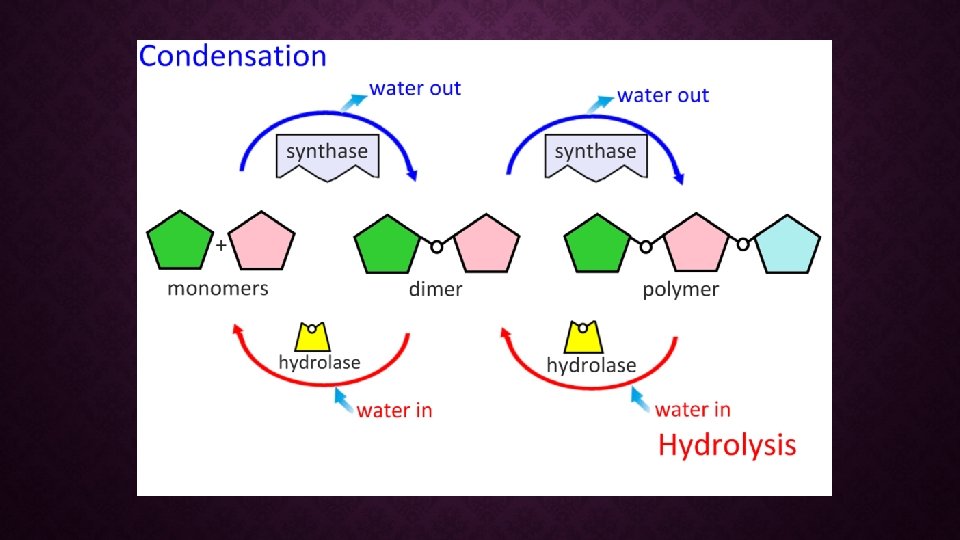
Anabolic! SYNTHESIS OF ORGANIC MOLECULES • Organic molecules are POLYMERS (chains) of subunits called MONOMERS. • Monomers are joined together through CONDENSATION REACTIONS • Also known as DEHYDRATION SYNTHESIS An H and an OH are removed, resulting in the loss of a water molecule.
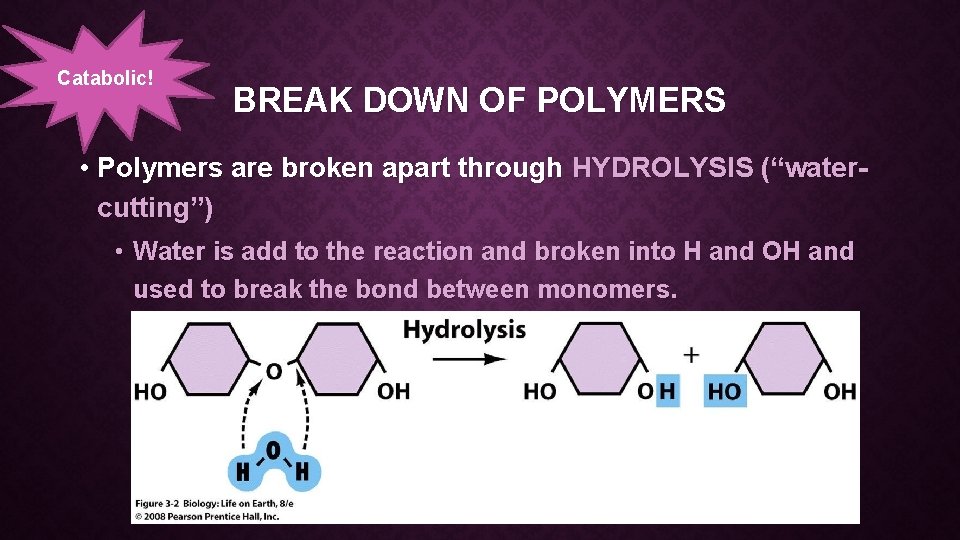
Catabolic! BREAK DOWN OF POLYMERS • Polymers are broken apart through HYDROLYSIS (“watercutting”) • Water is add to the reaction and broken into H and OH and used to break the bond between monomers.
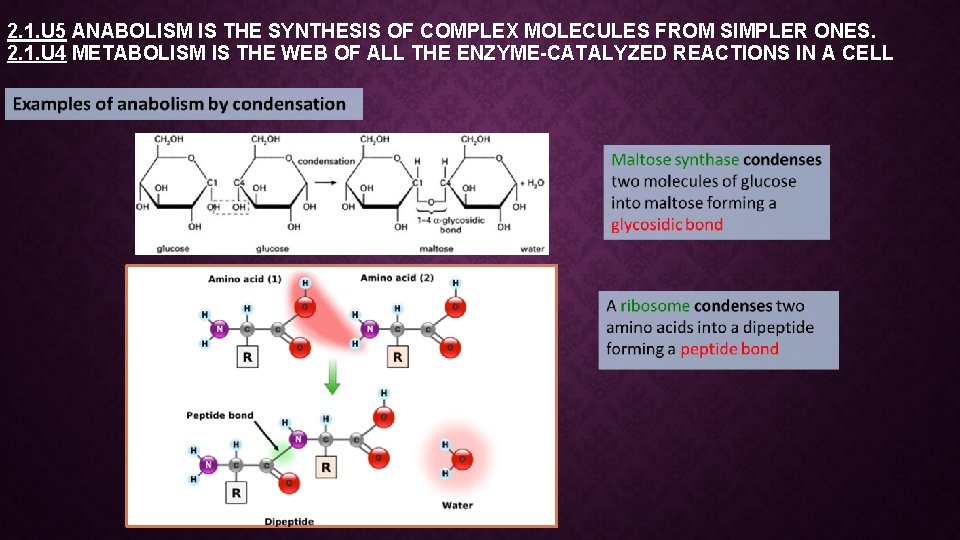
2. 1. U 5 ANABOLISM IS THE SYNTHESIS OF COMPLEX MOLECULES FROM SIMPLER ONES. 2. 1. U 4 METABOLISM IS THE WEB OF ALL THE ENZYME-CATALYZED REACTIONS IN A CELL
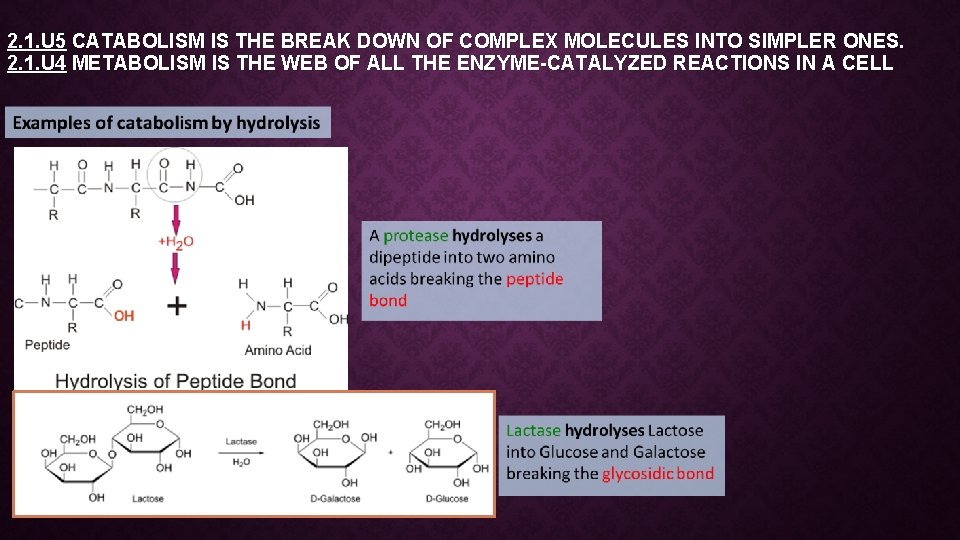
2. 1. U 5 CATABOLISM IS THE BREAK DOWN OF COMPLEX MOLECULES INTO SIMPLER ONES. 2. 1. U 4 METABOLISM IS THE WEB OF ALL THE ENZYME-CATALYZED REACTIONS IN A CELL
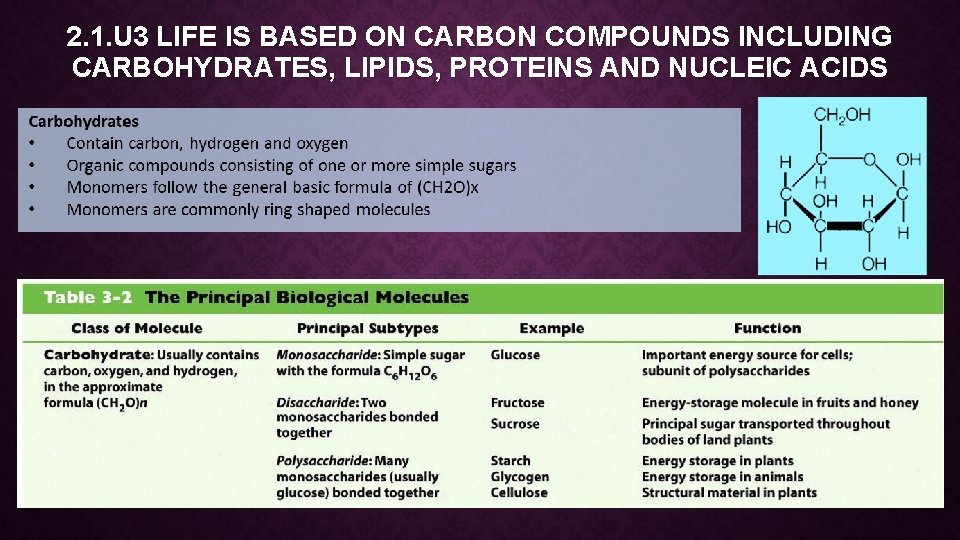
2. 1. U 3 LIFE IS BASED ON CARBON COMPOUNDS INCLUDING CARBOHYDRATES, LIPIDS, PROTEINS AND NUCLEIC ACIDS
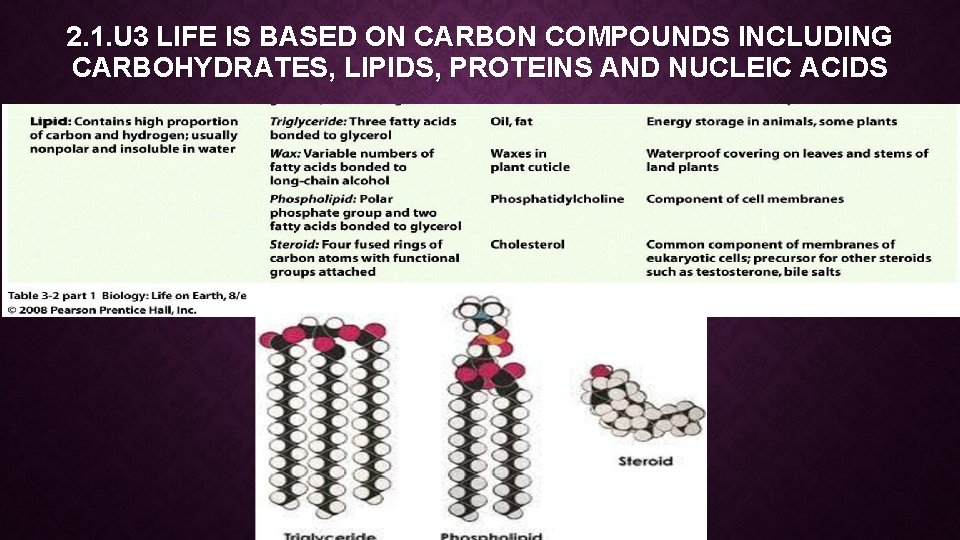
2. 1. U 3 LIFE IS BASED ON CARBON COMPOUNDS INCLUDING CARBOHYDRATES, LIPIDS, PROTEINS AND NUCLEIC ACIDS
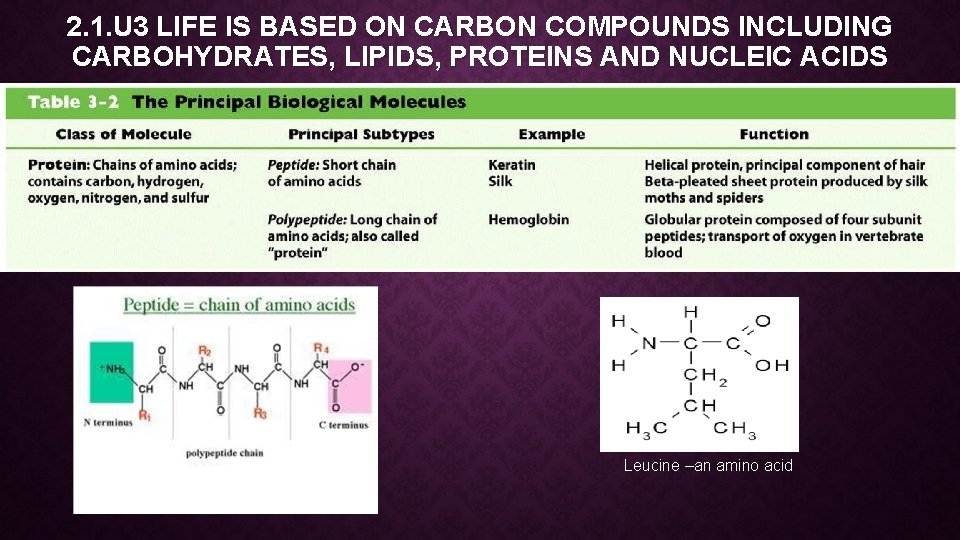
2. 1. U 3 LIFE IS BASED ON CARBON COMPOUNDS INCLUDING CARBOHYDRATES, LIPIDS, PROTEINS AND NUCLEIC ACIDS Leucine –an amino acid
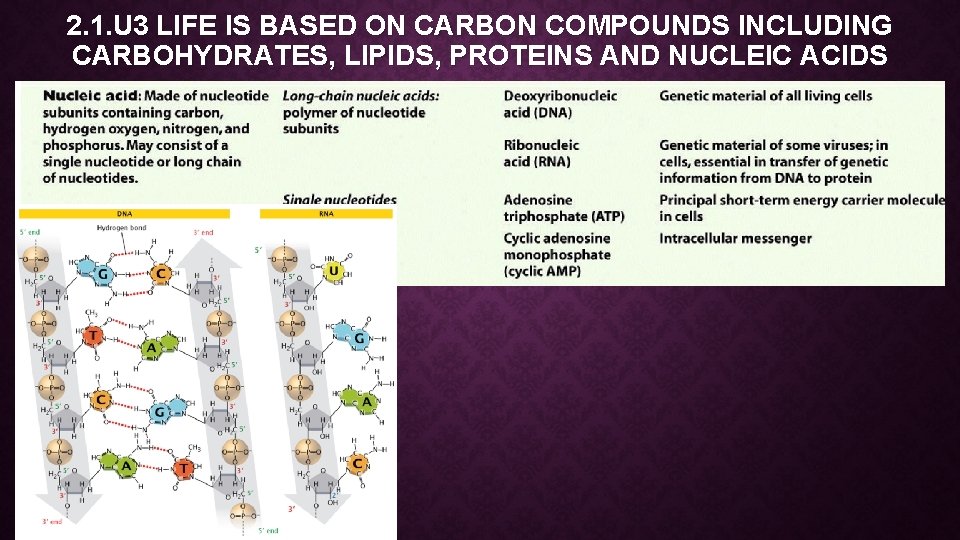
2. 1. U 3 LIFE IS BASED ON CARBON COMPOUNDS INCLUDING CARBOHYDRATES, LIPIDS, PROTEINS AND NUCLEIC ACIDS
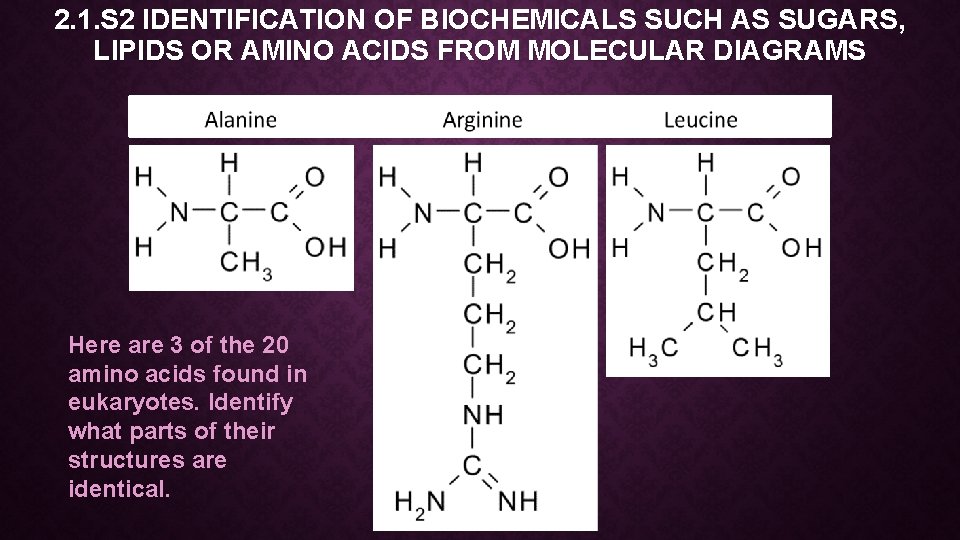
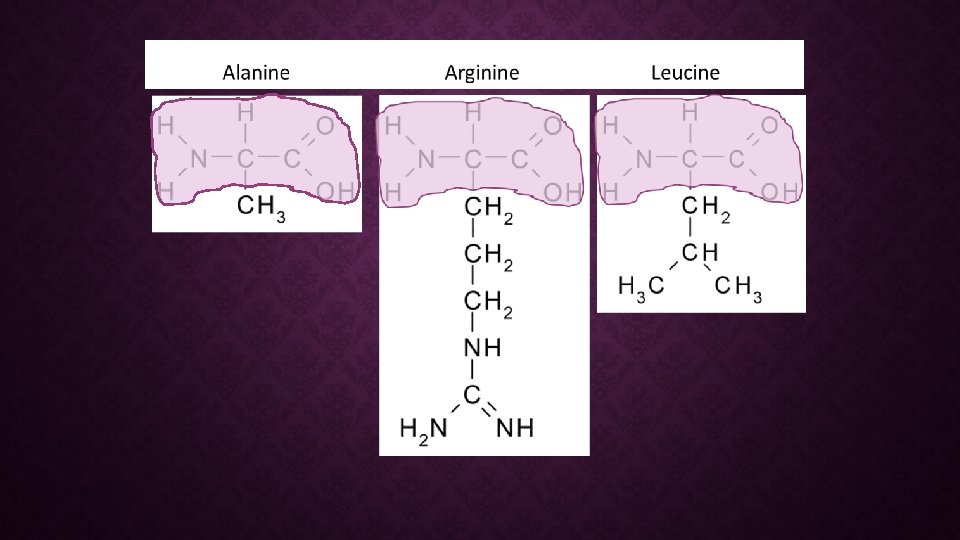
2. 1. S 2 IDENTIFICATION OF BIOCHEMICALS SUCH AS SUGARS, LIPIDS OR AMINO ACIDS FROM MOLECULAR DIAGRAMS Here are 3 of the 20 amino acids found in eukaryotes. Identify what parts of their structures are identical.
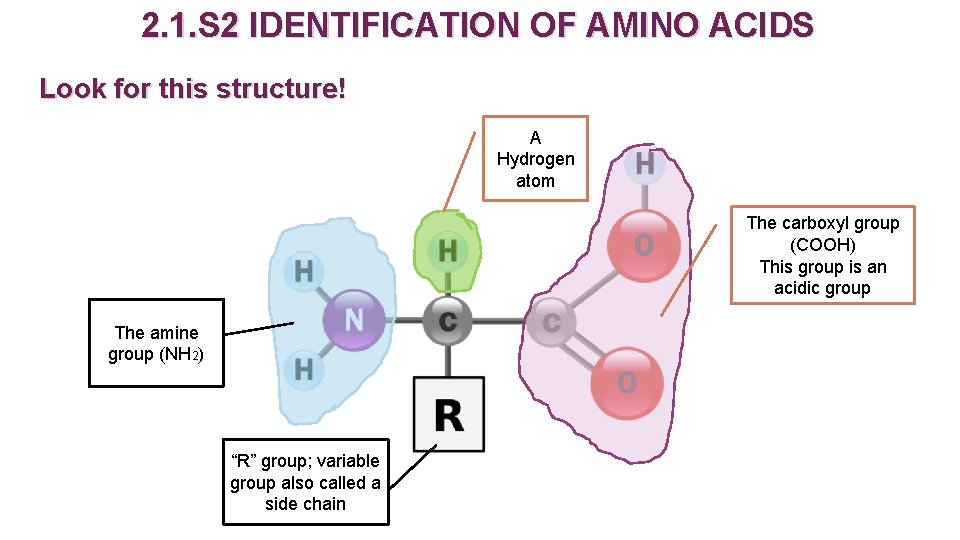
2. 1. S 2 IDENTIFICATION OF AMINO ACIDS Look for this structure! A Hydrogen atom The carboxyl group (COOH) This group is an acidic group The amine group (NH 2) “R” group; variable group also called a side chain
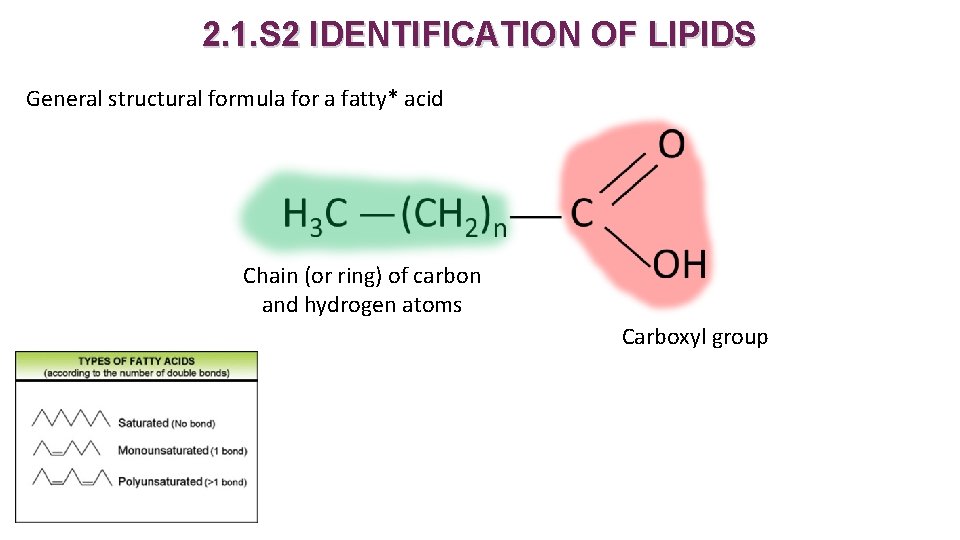
2. 1. S 2 IDENTIFICATION OF LIPIDS General structural formula for a fatty* acid Chain (or ring) of carbon and hydrogen atoms Carboxyl group
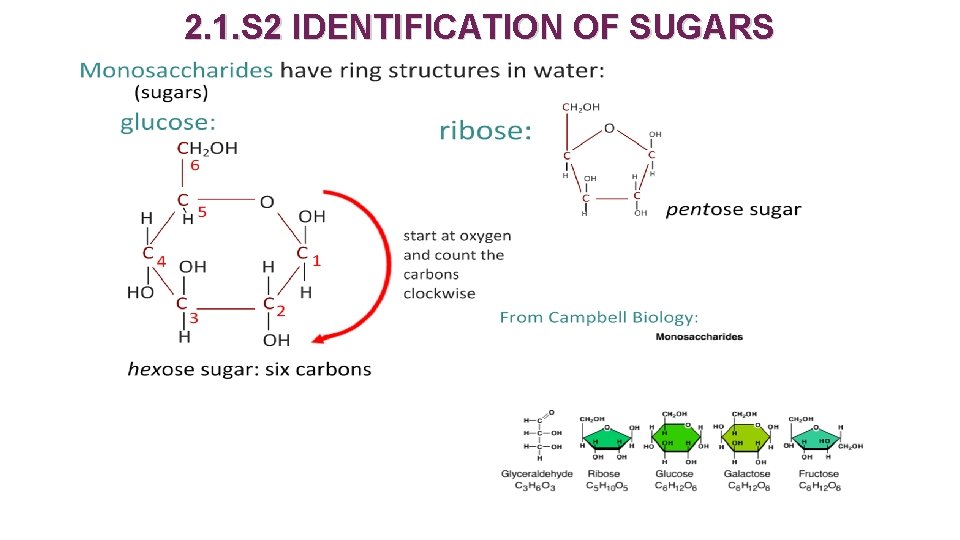
2. 1. S 2 IDENTIFICATION OF SUGARS
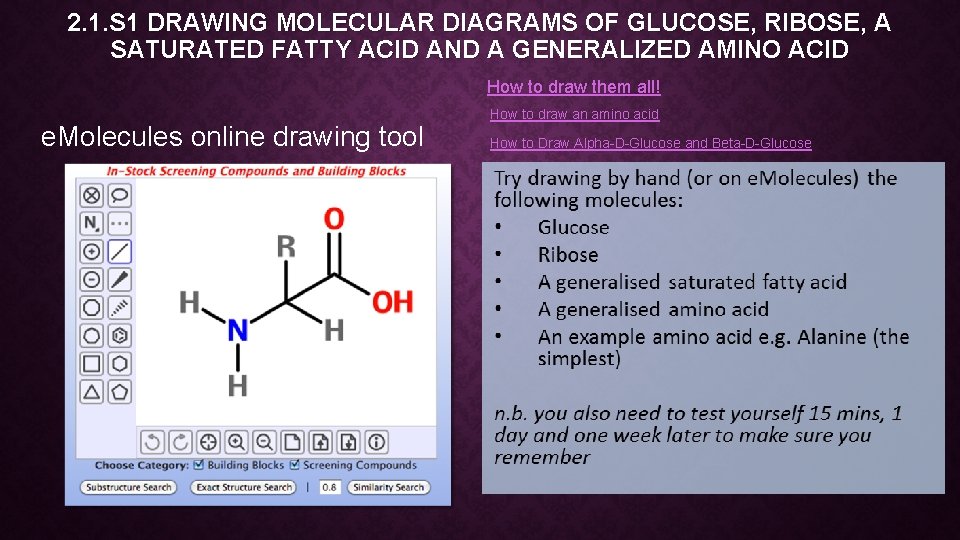
2. 1. S 1 DRAWING MOLECULAR DIAGRAMS OF GLUCOSE, RIBOSE, A SATURATED FATTY ACID AND A GENERALIZED AMINO ACID How to draw them all! e. Molecules online drawing tool How to draw an amino acid How to Draw Alpha-D-Glucose and Beta-D-Glucose
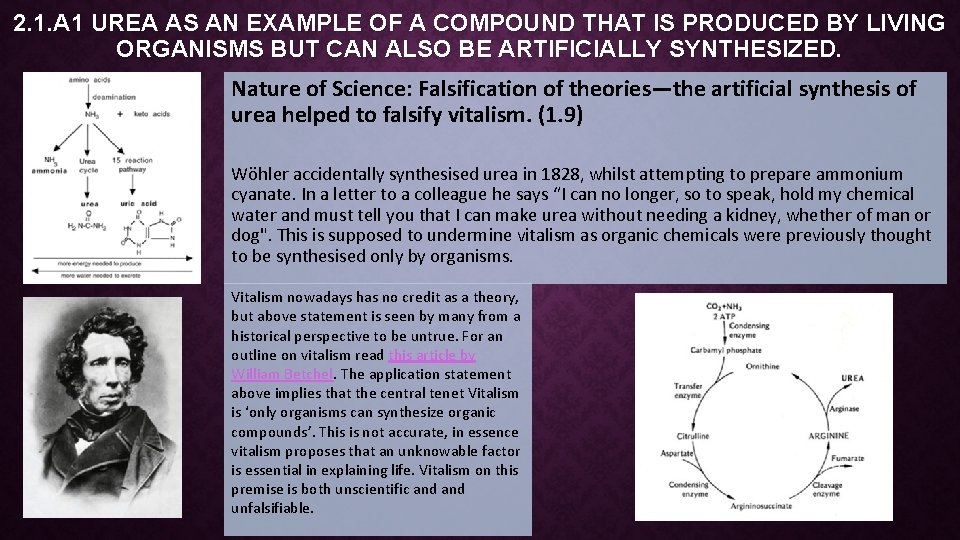
2. 1. A 1 UREA AS AN EXAMPLE OF A COMPOUND THAT IS PRODUCED BY LIVING ORGANISMS BUT CAN ALSO BE ARTIFICIALLY SYNTHESIZED. Nature of Science: Falsification of theories—the artificial synthesis of urea helped to falsify vitalism. (1. 9) Wöhler accidentally synthesised urea in 1828, whilst attempting to prepare ammonium cyanate. In a letter to a colleague he says “I can no longer, so to speak, hold my chemical water and must tell you that I can make urea without needing a kidney, whether of man or dog". This is supposed to undermine vitalism as organic chemicals were previously thought to be synthesised only by organisms. Vitalism nowadays has no credit as a theory, but above statement is seen by many from a historical perspective to be untrue. For an outline on vitalism read this article by William Betchel. The application statement above implies that the central tenet Vitalism is ‘only organisms can synthesize organic compounds’. This is not accurate, in essence vitalism proposes that an unknowable factor is essential in explaining life. Vitalism on this premise is both unscientific and unfalsifiable.
- Slides: 27