Unit 7 Chemical Reactions Physical Vs Chemical Change










- Slides: 43

Unit 7 Chemical Reactions

Physical Vs. Chemical Change • Physical changes= changes in form (phase), but not the identity of the substance ex: H 2 O (s) H 2 O (l) (* Starts as H 2 O and ends as H 2 O) • Chemical Change = Covalent/ ionic bonds break between reactants, producing new products. ex: H 2 O (l) H 2 (g)+ O 2 (g)

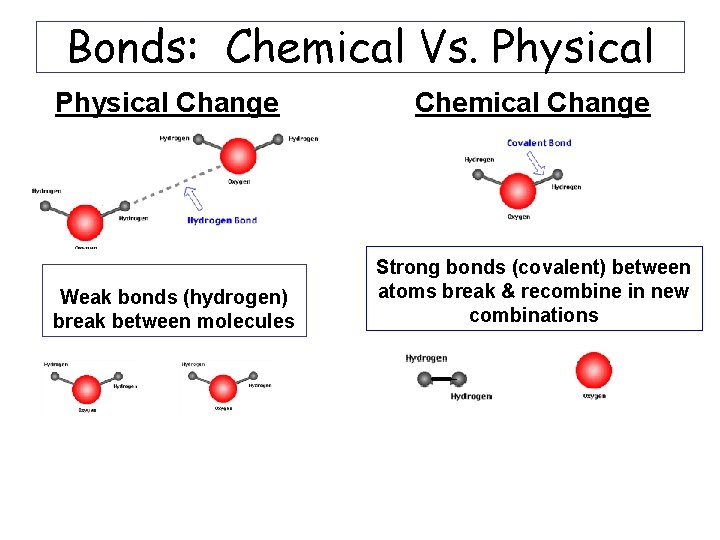
Bonds: Chemical Vs. Physical Change Weak bonds (hydrogen) break between molecules Chemical Change Strong bonds (covalent) between atoms break & recombine in new combinations

Indicators of a Chemical Rxn 1) 2) 3) 4) Light/ heat are produced Color Change Formation of a precipitate (solid) Production of a gas

Importance of a Chemical Equation § It’s a chemical sentence describing a chemical or physical change • Ex: HCl (aq) + Na. OH (aq) Na. Cl (aq) + H 2 O (l) Reactants Products Symbols used in Chemical Equations (s) = solid (l) = liquid (aq) = aqueous, a. k. a. dissolved in water → = yields/ results (g) = gas ↔ = reversible reaction = Reactions are heated There are many more symbols used too!

The charcoal used in a grill is basically carbon. The carbon reacts with oxygen to yield carbon dioxide. C + O 2 CO 2,

Naming & Formula Writing for Ionic and Covalent compounds Pgs 203 -215

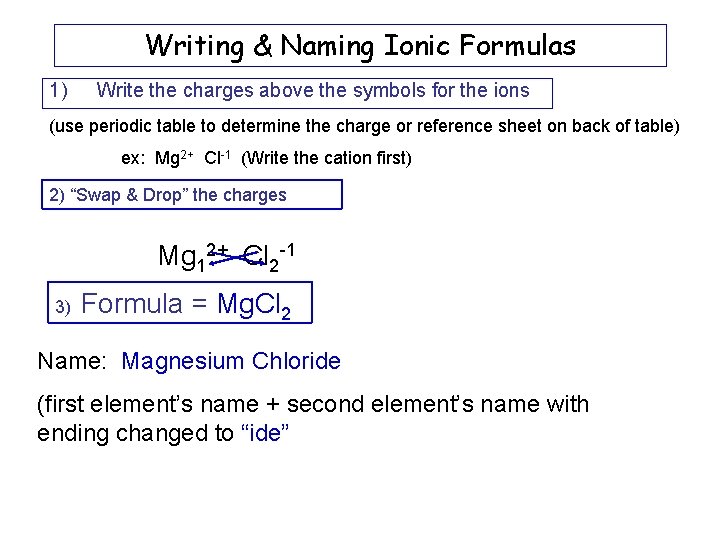
Writing & Naming Ionic Formulas 1) Write the charges above the symbols for the ions (use periodic table to determine the charge or reference sheet on back of table) ex: Mg 2+ Cl-1 (Write the cation first) 2) “Swap & Drop” the charges Mg 12+ Cl 2 -1 3) Formula = Mg. Cl 2 Name: Magnesium Chloride (first element’s name + second element’s name with ending changed to “ide”

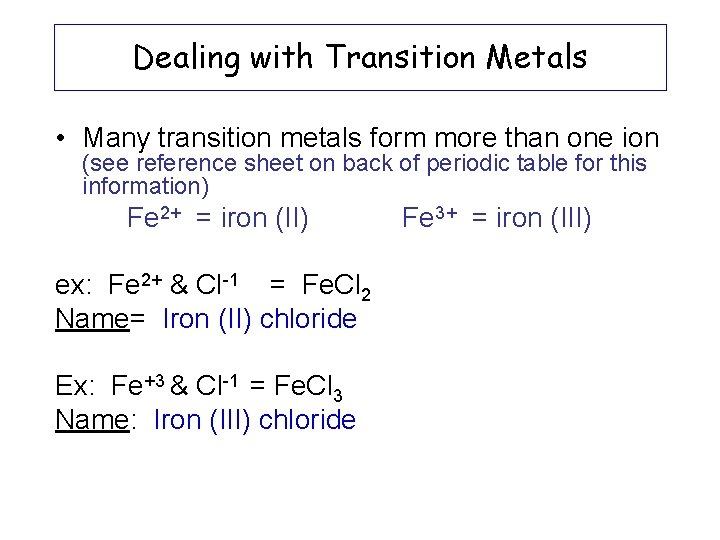
Dealing with Transition Metals • Many transition metals form more than one ion (see reference sheet on back of periodic table for this information) Fe 2+ = iron (II) ex: Fe 2+ & Cl-1 = Fe. Cl 2 Name= Iron (II) chloride Ex: Fe+3 & Cl-1 = Fe. Cl 3 Name: Iron (III) chloride Fe 3+ = iron (III)

Formulas & Naming (w/ Polyatomic Ions) (SO 4) = polyatomic ion -acts as an ion, but has more than one atom • Show formula for: Al bonded with SO 4 Al+3 SO 4 -2 Formula = Al 2(SO 4)3 Name: Aluminum sulfate

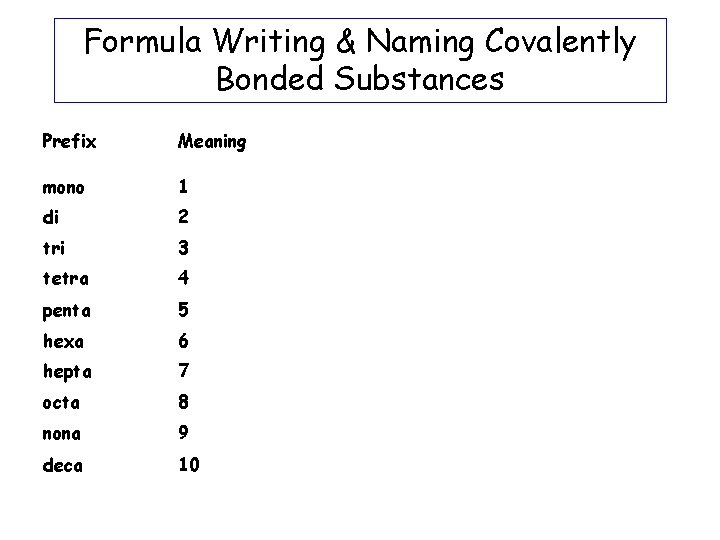
Formula Writing & Naming Covalently Bonded Substances Prefix Meaning mono 1 di 2 tri 3 tetra 4 penta 5 hexa 6 hepta 7 octa 8 nona 9 deca 10 Prefixes used in Covalently bonded molecules

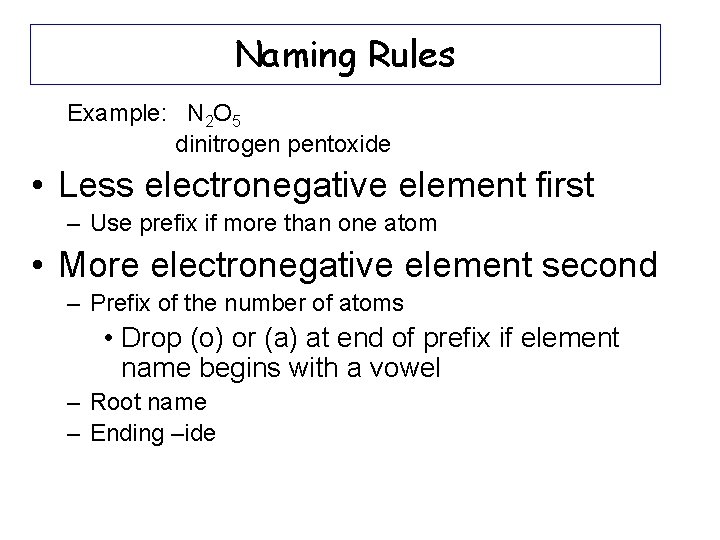
Naming Rules Example: N 2 O 5 dinitrogen pentoxide • Less electronegative element first – Use prefix if more than one atom • More electronegative element second – Prefix of the number of atoms • Drop (o) or (a) at end of prefix if element name begins with a vowel – Root name – Ending –ide

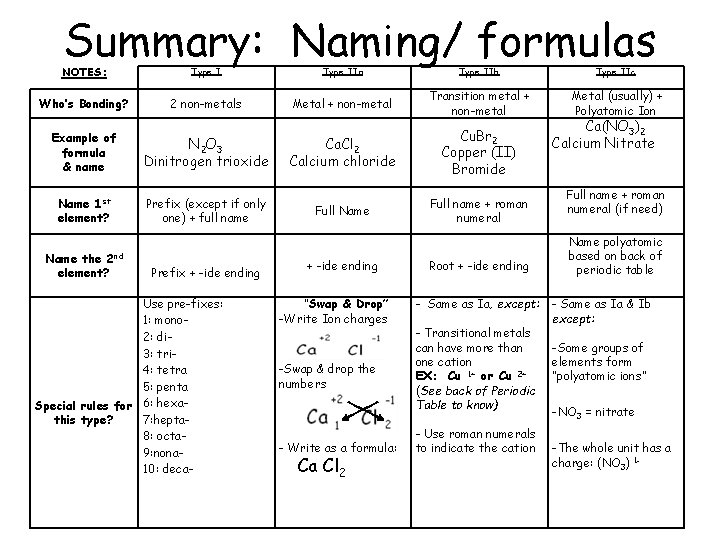
Summary: Naming/ formulas NOTES: Type IIa Type IIb Type IIc Who’s Bonding? 2 non-metals Metal + non-metal Transition metal + non-metal Metal (usually) + Polyatomic Ion Ca. Cl 2 Calcium chloride Cu. Br 2 Copper (II) Bromide Full Name Full name + roman numeral Example of formula & name N 2 O 3 Dinitrogen trioxide Name 1 st element? Prefix (except if only one) + full name 2 nd Name the element? Prefix + -ide ending Use pre-fixes: 1: mono 2: di 3: tri 4: tetra 5: penta Special rules for 6: hexa 7: heptathis type? 8: octa 9: nona 10: deca- + -ide ending “Swap & Drop” -Write Ion charges -Swap & drop the numbers - Write as a formula: Ca Cl 2 Root + -ide ending - Same as Ia, except: - Transitional metals can have more than one cation EX: Cu 1+ or Cu 2+ (See back of Periodic Table to know) - Use roman numerals to indicate the cation Ca(NO 3)2 Calcium Nitrate Full name + roman numeral (if need) Name polyatomic based on back of periodic table - Same as Ia & Ib except: -Some groups of elements form “polyatomic ions” -NO 3 = nitrate -The whole unit has a charge: (NO 3) 1 -

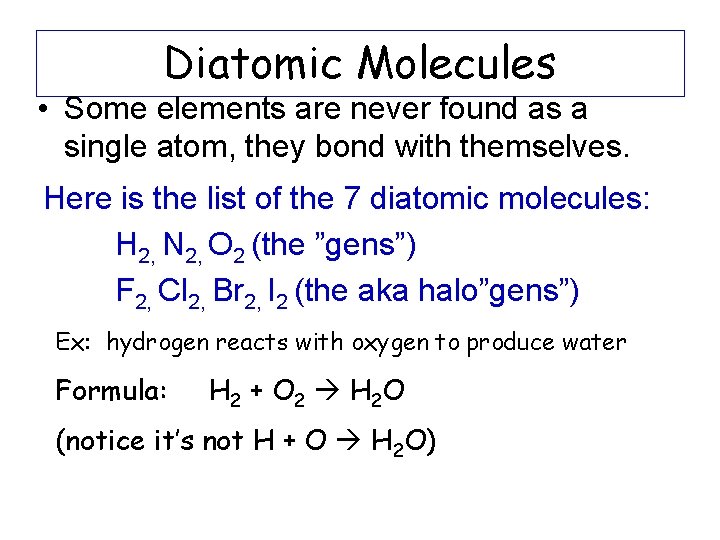
Diatomic Molecules • Some elements are never found as a single atom, they bond with themselves. Here is the list of the 7 diatomic molecules: H 2, N 2, O 2 (the ”gens”) F 2, Cl 2, Br 2, I 2 (the aka halo”gens”) Ex: hydrogen reacts with oxygen to produce water Formula: H 2 + O 2 H 2 O (notice it’s not H + O H 2 O)

Types of Chemical Reactions

Types of Chemical Reactions There are 5 types of chemical reactions : 1. 2. 3. 4. 5. Synthesis reactions Decomposition reactions Single replacement reactions Double replacement reactions Combustion reactions You need to be able to identify the type of reaction and predict the product(s)

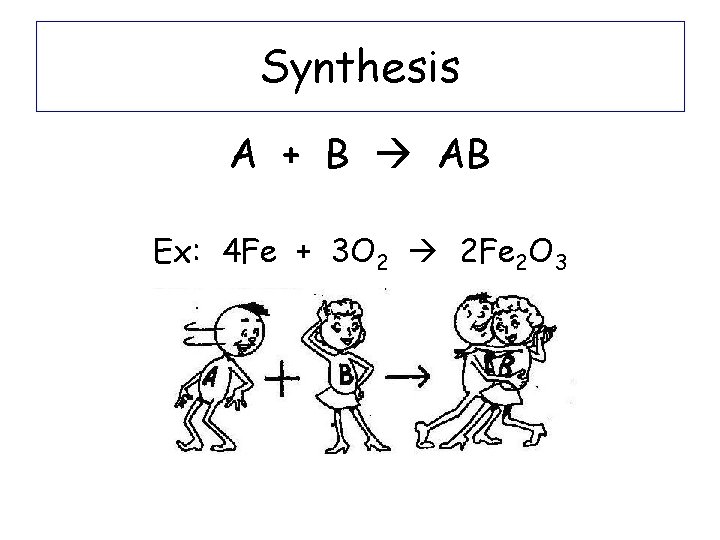
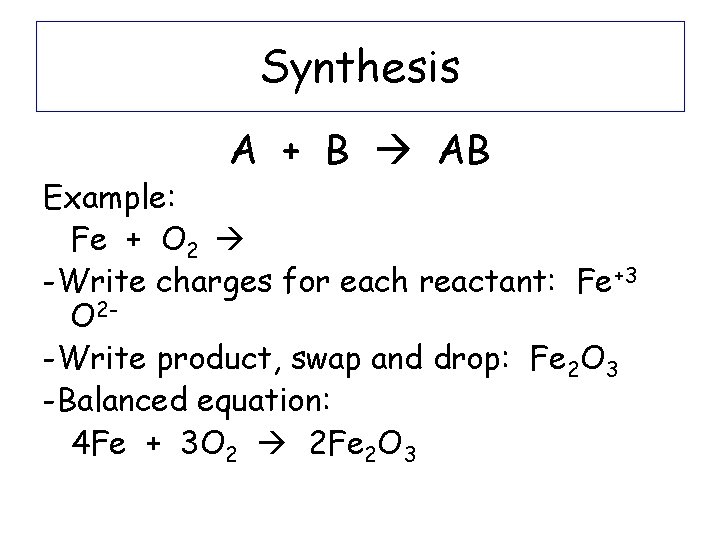
Synthesis A + B AB Ex: 4 Fe + 3 O 2 2 Fe 2 O 3

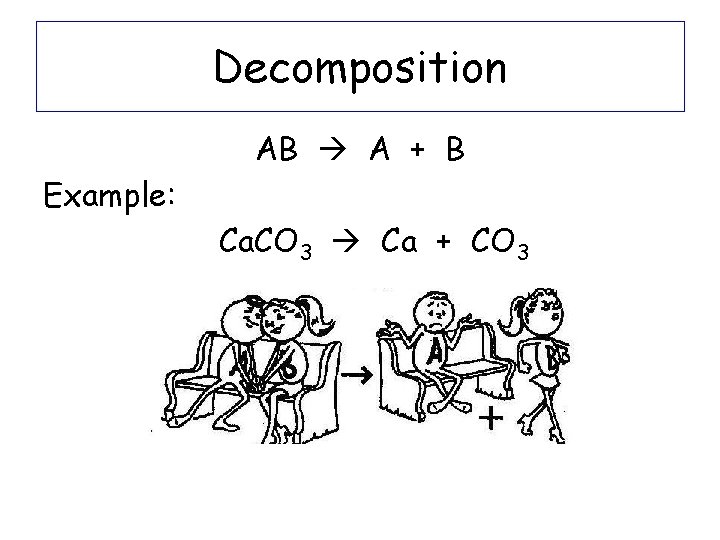
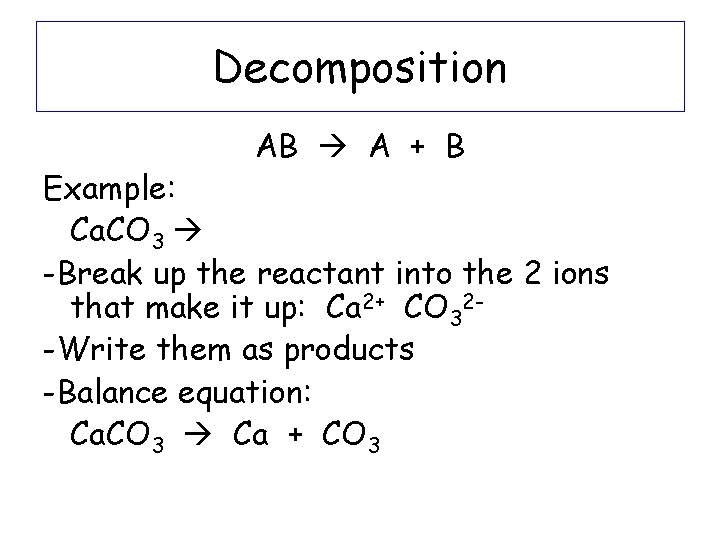
Decomposition AB A + B Example: Ca. CO 3 Ca + CO 3

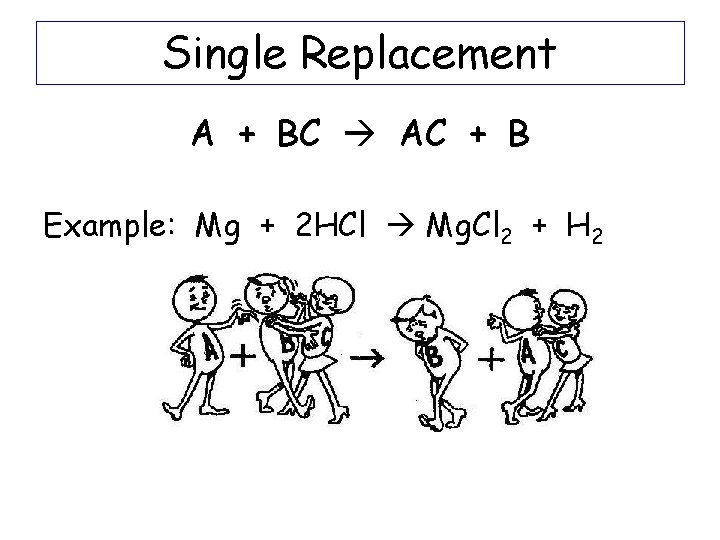
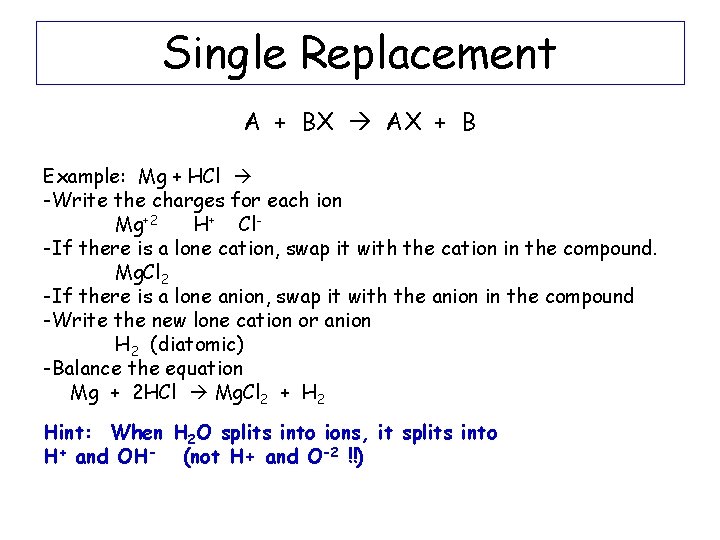
Single Replacement A + BC AC + B Example: Mg + 2 HCl Mg. Cl 2 + H 2

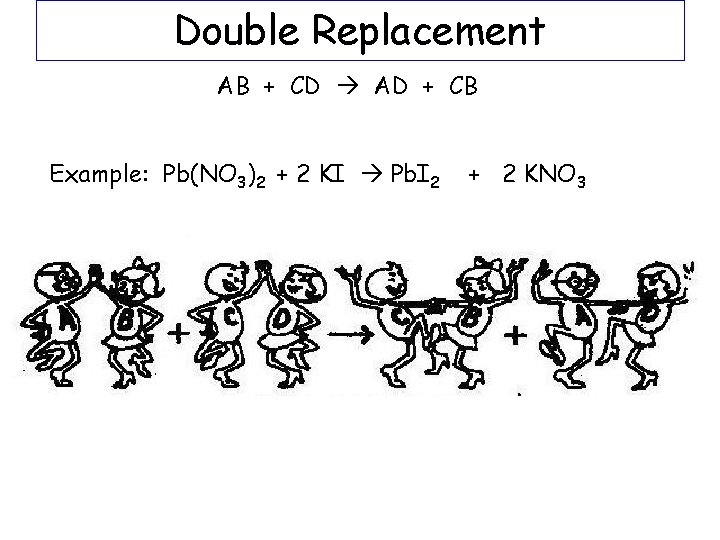
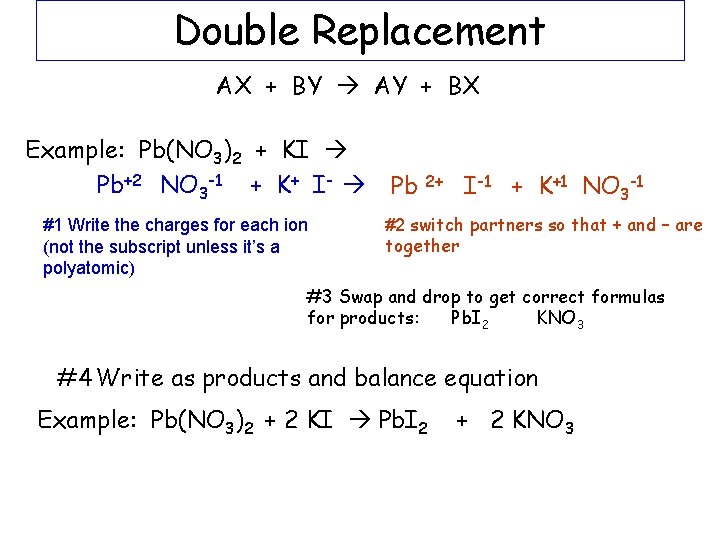
Double Replacement AB + CD AD + CB Example: Pb(NO 3)2 + 2 KI Pb. I 2 + 2 KNO 3

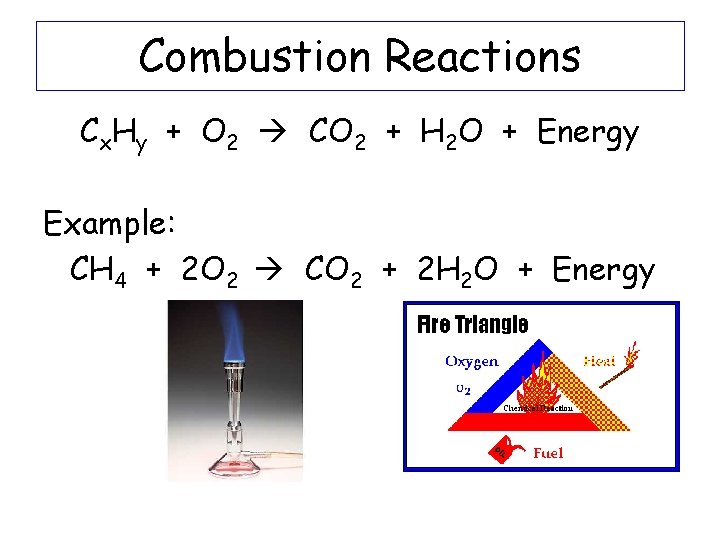
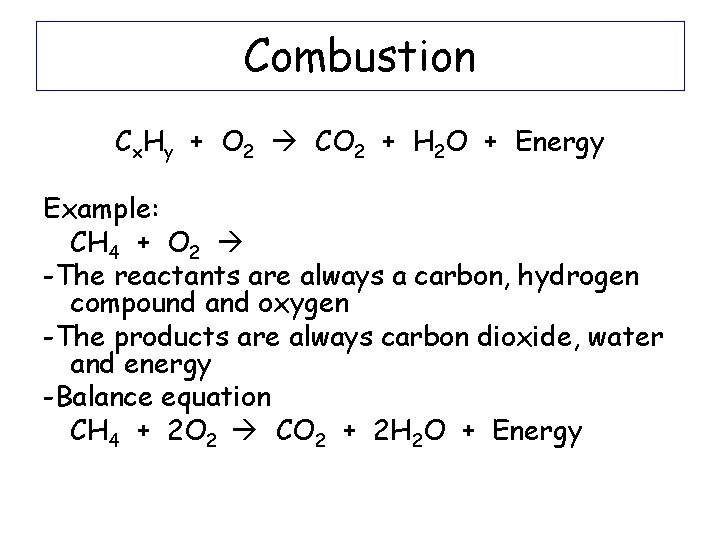
Combustion Reactions Cx. Hy + O 2 CO 2 + H 2 O + Energy Example: CH 4 + 2 O 2 CO 2 + 2 H 2 O + Energy

Balancing Chemical Equations

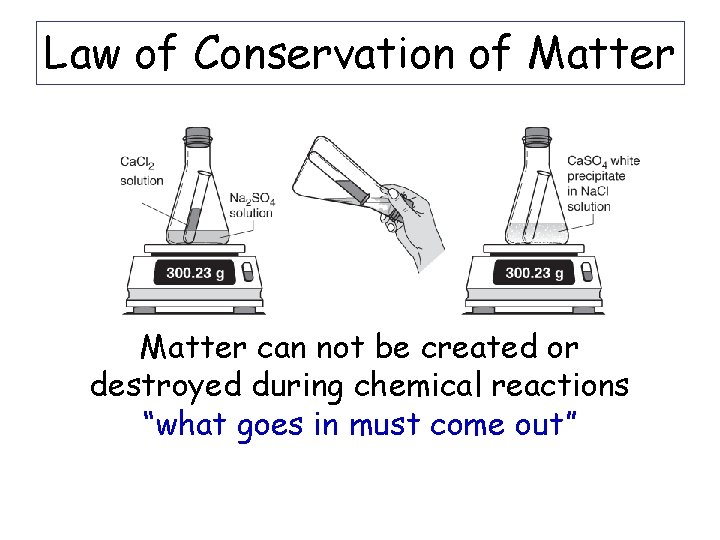
Law of Conservation of Matter can not be created or destroyed during chemical reactions “what goes in must come out”

Law of Conservation of Matter # of atoms that go in a chemical reaction MUST come out” Unbalanced EX: C 6 H 12 O 6 + O 2 CO 2 + H 2 O 6 C’s go in & only 1 C comes out (H’s and O’s don’t add up either!) Not possible! § We balance chemical equations to adhere to the Law of conservation of matter. EX: C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O (now it’s balanced) -

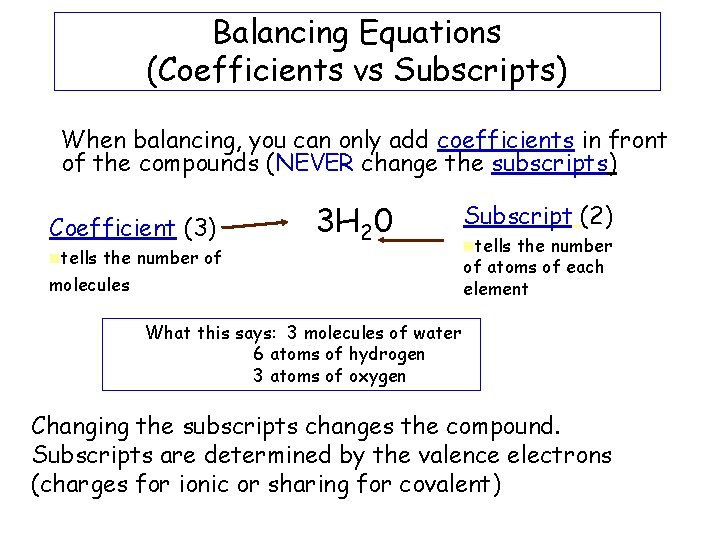
Balancing Equations (Coefficients vs Subscripts) When balancing, you can only add coefficients in front of the compounds (NEVER change the subscripts) Coefficient (3) ntells the number of 3 H 20 molecules Subscript (2) ntells the number of atoms of each element What this says: 3 molecules of water 6 atoms of hydrogen 3 atoms of oxygen Changing the subscripts changes the compound. Subscripts are determined by the valence electrons (charges for ionic or sharing for covalent)

Steps to balancing chemical equations: 1) Balance one element at a time. Working left to right, find the number of atoms for each element on the left side and compare those against the number of the atoms of the same element on the right side. Helpful hint: Never start by balancing the H’s or O’s. Save H for next to last and O until last. 2) Place coefficients in front of formulas so that the left side has the same number of atoms as the right side. Helpful hint: Polyatomic ions that appear on both sides of the equation should be balanced as independent units 3) Double check your math to make sure you’ve balanced everything correctly.

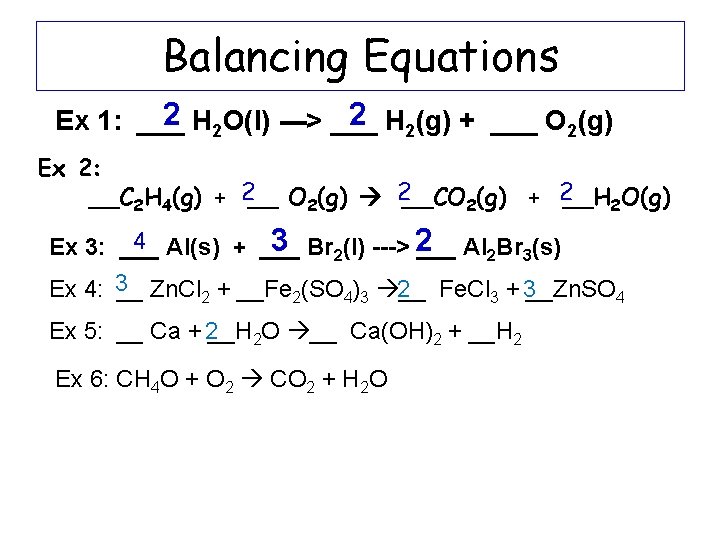
Balancing Equations 2 H 2 O(l) ---> ___ 2 H 2(g) + ___ O 2(g) Ex 1: ___ Ex 2: __C 2 H 4(g) + 2__ O 2(g) 2__CO 2(g) + 2__H 2 O(g) 4 Al(s) + ___ 3 Br 2(l) ---> 2 Ex 3: ___ Al 2 Br 3(s) Ex 4: 3__ Zn. Cl 2 + __Fe 2(SO 4)3 __ 2 Fe. Cl 3 + 3__Zn. SO 4 Ex 5: __ Ca + 2__H 2 O __ Ca(OH)2 + __H 2 Ex 6: CH 4 O + O 2 CO 2 + H 2 O

Predicting Products & Will Reaction Work?

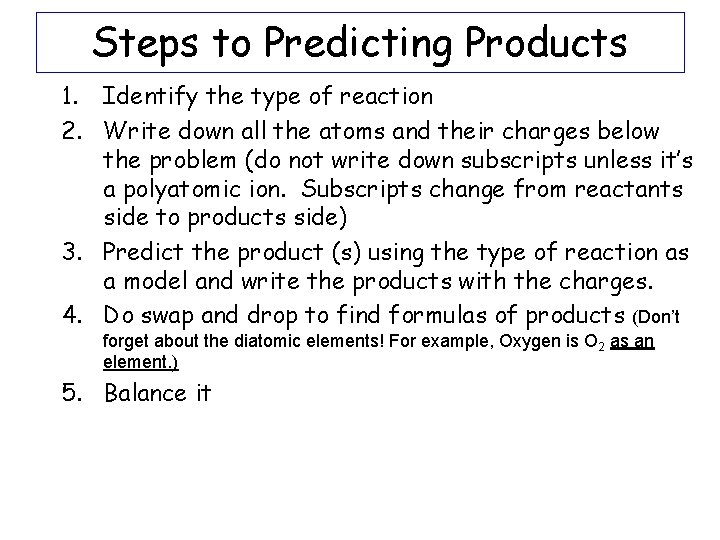
Steps to Predicting Products 1. Identify the type of reaction 2. Write down all the atoms and their charges below the problem (do not write down subscripts unless it’s a polyatomic ion. Subscripts change from reactants side to products side) 3. Predict the product (s) using the type of reaction as a model and write the products with the charges. 4. Do swap and drop to find formulas of products (Don’t forget about the diatomic elements! For example, Oxygen is O 2 as an element. ) 5. Balance it

Synthesis A + B AB Example: Fe + O 2 -Write charges for each reactant: Fe+3 O 2 -Write product, swap and drop: Fe 2 O 3 -Balanced equation: 4 Fe + 3 O 2 2 Fe 2 O 3

Decomposition AB A + B Example: Ca. CO 3 -Break up the reactant into the 2 ions that make it up: Ca 2+ CO 32 -Write them as products -Balance equation: Ca. CO 3 Ca + CO 3

Single Replacement A + BX AX + B Example: Mg + HCl -Write the charges for each ion Mg+2 H+ Cl-If there is a lone cation, swap it with the cation in the compound. Mg. Cl 2 -If there is a lone anion, swap it with the anion in the compound -Write the new lone cation or anion H 2 (diatomic) -Balance the equation Mg + 2 HCl Mg. Cl 2 + H 2 Hint: When H 2 O splits into ions, it splits into H+ and OH- (not H+ and O-2 !!)

Double Replacement AX + BY AY + BX Example: Pb(NO 3)2 + KI Pb+2 NO 3 -1 + K+ I- #1 Write the charges for each ion (not the subscript unless it’s a polyatomic) Pb 2+ I-1 + K+1 NO 3 -1 #2 switch partners so that + and – are together #3 Swap and drop to get correct formulas for products: Pb. I 2 KNO 3 #4 Write as products and balance equation Example: Pb(NO 3)2 + 2 KI Pb. I 2 + 2 KNO 3

Combustion Cx. Hy + O 2 CO 2 + H 2 O + Energy Example: CH 4 + O 2 -The reactants are always a carbon, hydrogen compound and oxygen -The products are always carbon dioxide, water and energy -Balance equation CH 4 + 2 O 2 CO 2 + 2 H 2 O + Energy

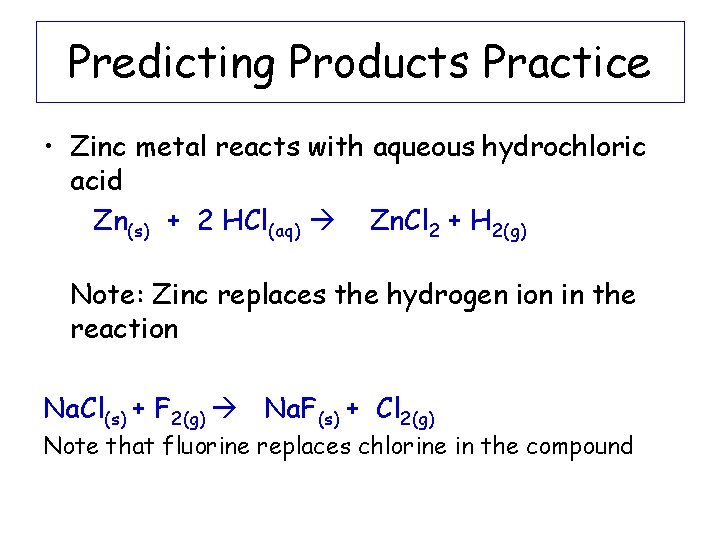
Predicting Products Practice • Zinc metal reacts with aqueous hydrochloric acid Zn(s) + 2 HCl(aq) Zn. Cl 2 + H 2(g) Note: Zinc replaces the hydrogen ion in the reaction Na. Cl(s) + F 2(g) Na. F(s) + Cl 2(g) Note that fluorine replaces chlorine in the compound

Will a Reaction Occur? n. Just because a reaction works out on paper, does not mean it would actually happen in a test tube. • 3 reactions ALWAYS work: – Combustion, Synthesis, and Decomposition Single replacement & Double replacement reactions do not always work. - You need more information for each type of reaction to know for sure if a reaction will take place.

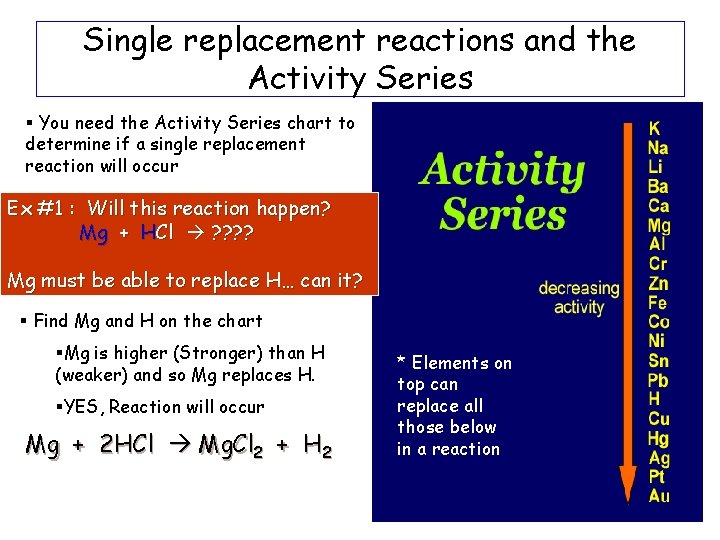
Single replacement reactions and the Activity Series § You need the Activity Series chart to determine if a single replacement reaction will occur Ex #1 : Will this reaction happen? Mg + HCl ? ? Mg must be able to replace H… can it? § Find Mg and H on the chart §Mg is higher (Stronger) than H (weaker) and so Mg replaces H. §YES, Reaction will occur Mg + 2 HCl Mg. Cl 2 + H 2 * Elements on top can replace all those below in a reaction

Single Replacement Reactions Practice Problems Will this reaction occur? Mg + Na. Cl ? ? ? Mg is trying to kick out Na… can it? -Na is higher on the chart than Mg so Na will not be kicked out by Mg. We write: Mg + Na. Cl NO REACTION

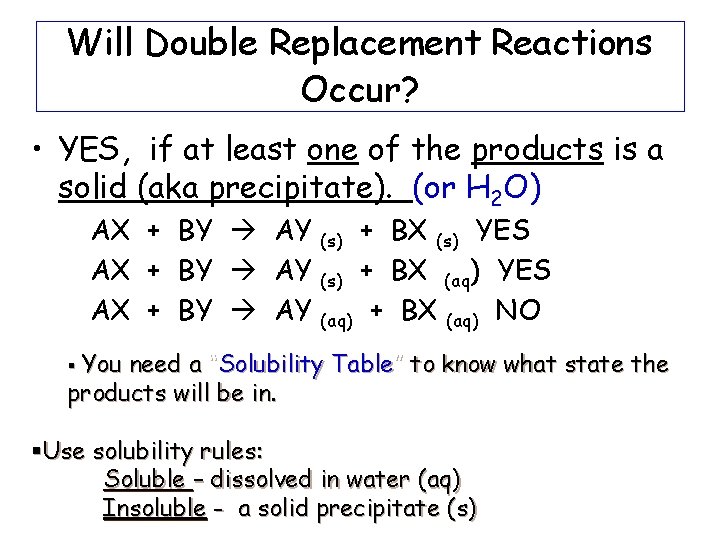
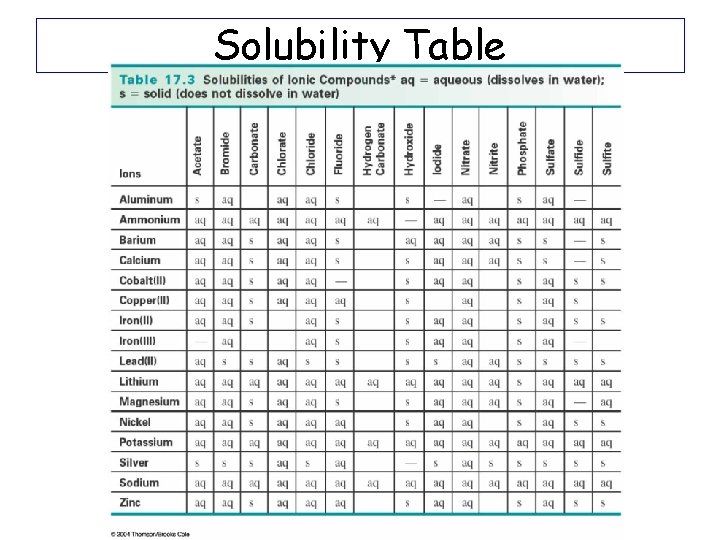
Will Double Replacement Reactions Occur? • YES, if at least one of the products is a solid (aka precipitate). (or H 2 O) AX + BY AY + BX (s) YES (s) + BX (aq) YES (aq) + BX (aq) NO (s) § You need a “Solubility Table” to know what state the products will be in. §Use solubility rules: Soluble – dissolved in water (aq) Insoluble - a solid precipitate (s)

Solubility Table The study of quantities of materials consumed and produced in chemical reactions.

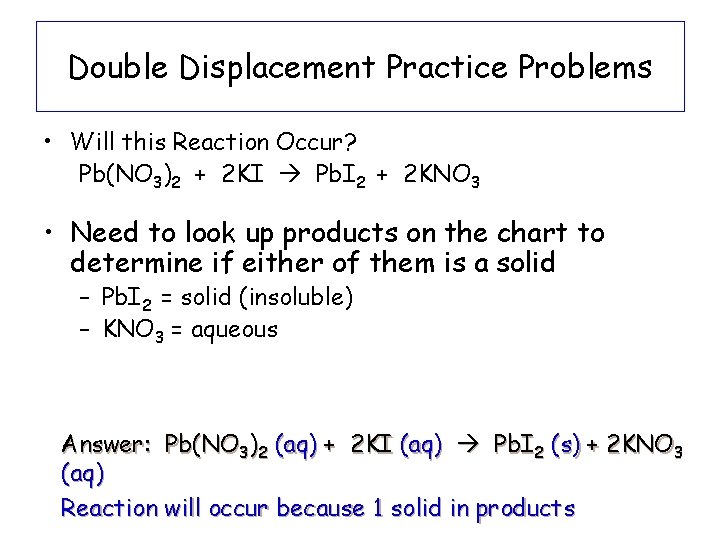
Double Displacement Practice Problems • Will this Reaction Occur? Pb(NO 3)2 + 2 KI Pb. I 2 + 2 KNO 3 • Need to look up products on the chart to determine if either of them is a solid – Pb. I 2 = solid (insoluble) – KNO 3 = aqueous Answer: Pb(NO 3)2 (aq) + 2 KI (aq) Pb. I 2 (s) + 2 KNO 3 (aq) Reaction will occur because 1 solid in products

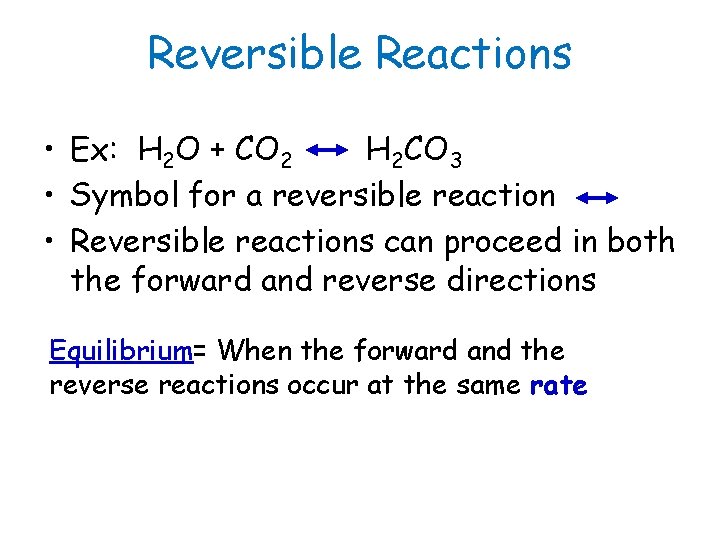
Reversible Reactions • Ex: H 2 O + CO 2 H 2 CO 3 • Symbol for a reversible reaction • Reversible reactions can proceed in both the forward and reverse directions Equilibrium= When the forward and the reverse reactions occur at the same rate

funny