Unit 7 Chemical Equilibrium Le Chteliers Principle Le
Unit 7: Chemical Equilibrium Le Châtelier’s Principle

Le Châtelier’s Principle l Le Châtelier’s Principle: Once a reaction has reached equilibrium, if you apply a stress to the system, the system will respond to regain equilibrium l Possible stresses include changes in: l l l concentration pressure temperature Video 1 and Video 2
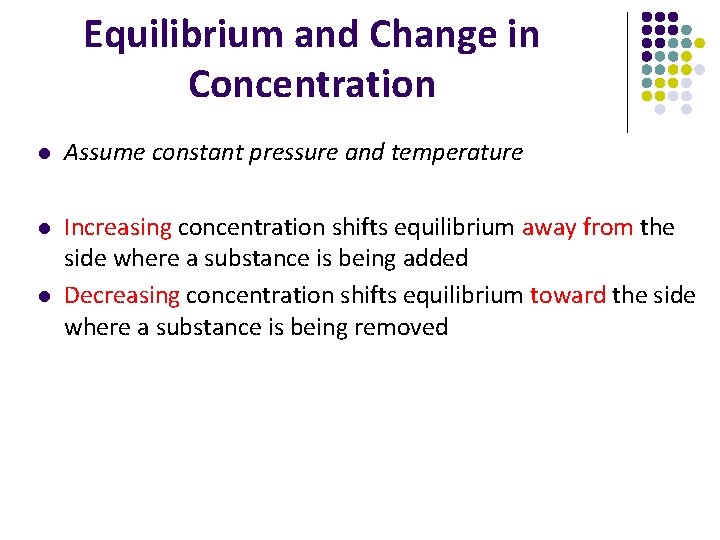
Equilibrium and Change in Concentration l Assume constant pressure and temperature l Increasing concentration shifts equilibrium away from the side where a substance is being added Decreasing concentration shifts equilibrium toward the side where a substance is being removed l

Equilibrium and Change in Concentration Example N 2 (g) + 3 H 2 (g) ↔ 2 NH 3 (g) If we add H 2 which way will equilibrium shift? l To the right – More NH 3 will form and N 2 will be used up (less N 2)
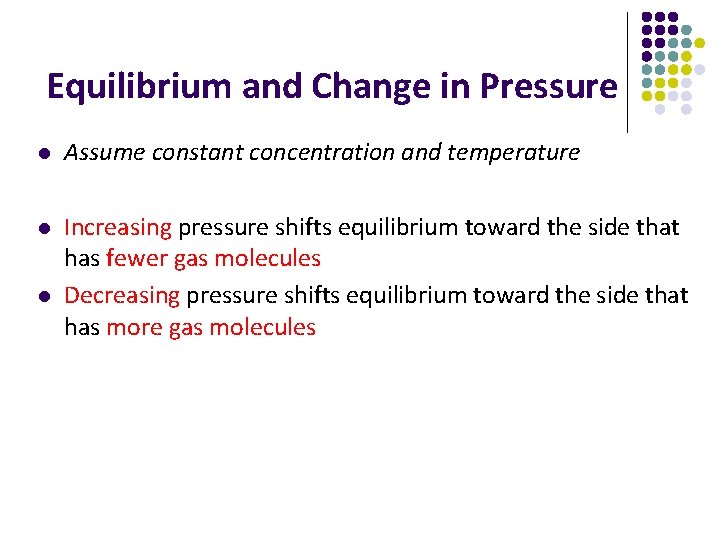
Equilibrium and Change in Pressure l Assume constant concentration and temperature l Increasing pressure shifts equilibrium toward the side that has fewer gas molecules Decreasing pressure shifts equilibrium toward the side that has more gas molecules l

Equilibrium and Change in Pressure Example N 2 (g) + 3 H 2 (g) ↔ 2 NH 3 (g) If we increase the pressure, which way will equilibrium shift? l To the right – More NH 3 will form
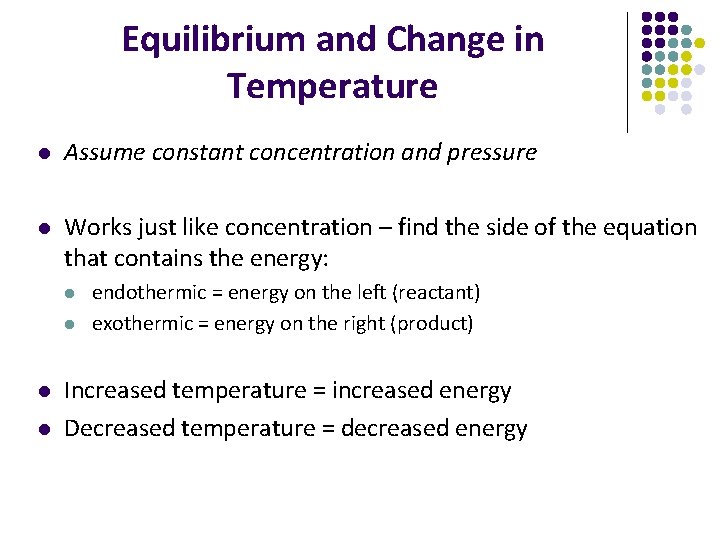
Equilibrium and Change in Temperature l Assume constant concentration and pressure l Works just like concentration – find the side of the equation that contains the energy: l l endothermic = energy on the left (reactant) exothermic = energy on the right (product) Increased temperature = increased energy Decreased temperature = decreased energy
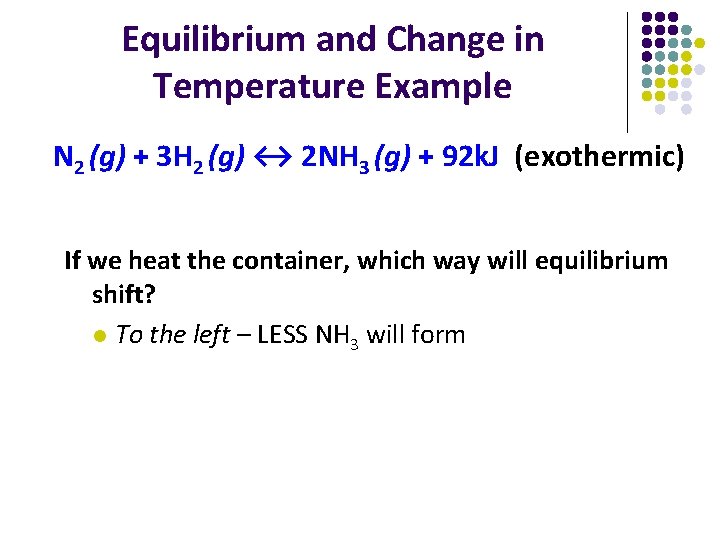
Equilibrium and Change in Temperature Example N 2 (g) + 3 H 2 (g) ↔ 2 NH 3 (g) + 92 k. J (exothermic) If we heat the container, which way will equilibrium shift? l To the left – LESS NH 3 will form
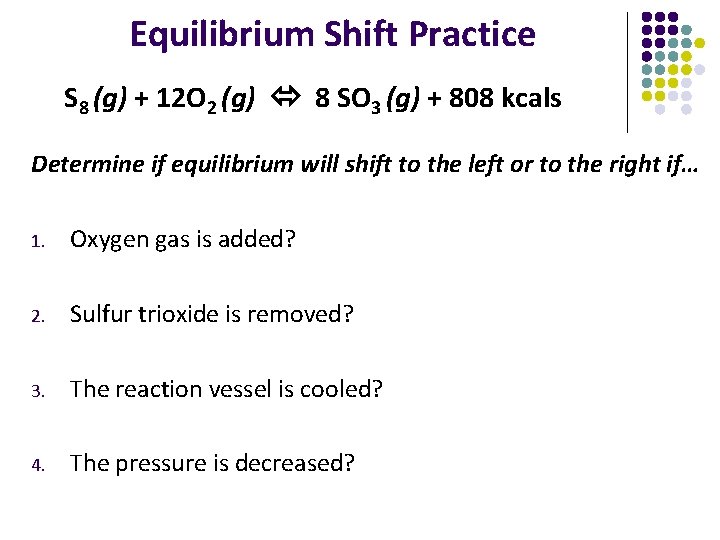
Equilibrium Shift Practice S 8 (g) + 12 O 2 (g) 8 SO 3 (g) + 808 kcals Determine if equilibrium will shift to the left or to the right if… 1. Oxygen gas is added? 2. Sulfur trioxide is removed? 3. The reaction vessel is cooled? 4. The pressure is decreased?
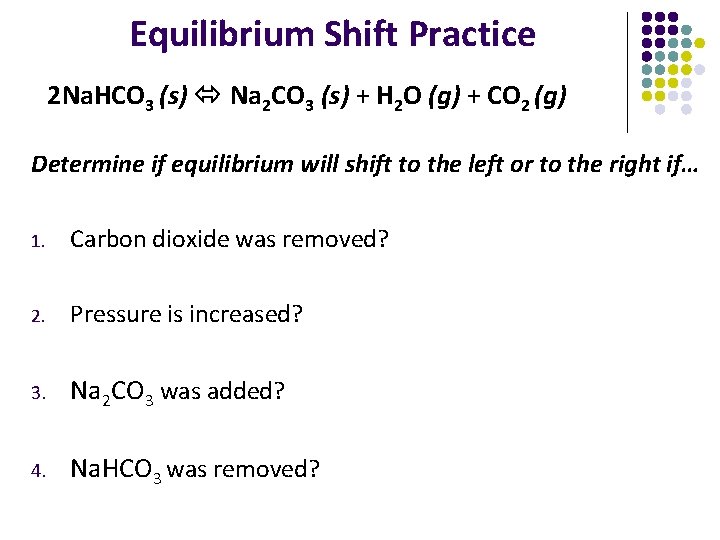
Equilibrium Shift Practice 2 Na. HCO 3 (s) Na 2 CO 3 (s) + H 2 O (g) + CO 2 (g) Determine if equilibrium will shift to the left or to the right if… 1. Carbon dioxide was removed? 2. Pressure is increased? 3. Na 2 CO 3 was added? 4. Na. HCO 3 was removed?
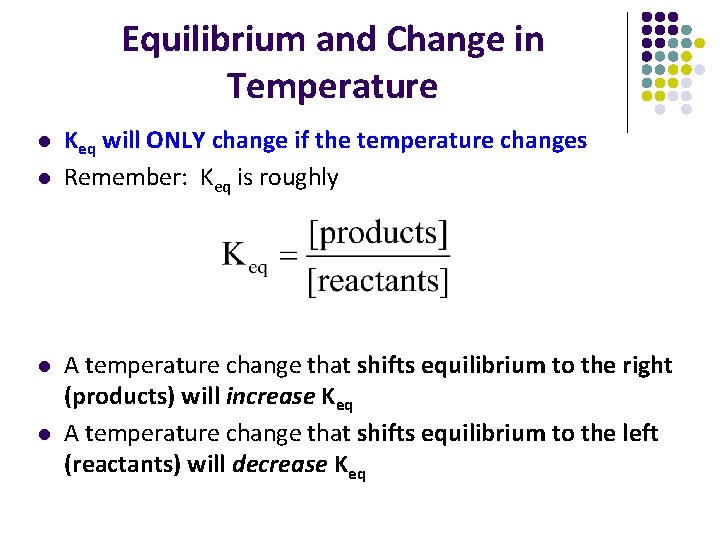
Equilibrium and Change in Temperature l l Keq will ONLY change if the temperature changes Remember: Keq is roughly A temperature change that shifts equilibrium to the right (products) will increase Keq A temperature change that shifts equilibrium to the left (reactants) will decrease Keq


Equilibrium Visualizations l http: //www. chem. arizona. edu/~jpollard/fido/fid o. html

Equilibrium Book Problems l l Read Ch. 15 (some parts we won’t cover) Assigned: 15. 2, 15. 4, 15. 6, 15. 9, 15. 16, 15. 52
- Slides: 14