Unit 7 1 Naming Molecular Compounds Teacher Dr




- Slides: 12

Unit 7. 1 Naming Molecular Compounds Teacher: Dr. Van Der Sluys

Objectives • Naming molecular compounds – Using prefixes – Stock naming system

Prefixes • A prefix is a morpheme (portion of a word) that comes at the beginning of the word and has a specific meaning Mono- one Penta- five Di- two Hexa- six Tri- three Hepta- seven Tetra- four Octa- eight

Naming Molecular Compounds with Prefixes 1. Use a prefix to indicate how many atoms there are of the first element in the formula, except if there is only one atom of the first element, then do not use the prefix mono-. 2. After the prefix, use the name of the first element in the formula. 3. Use a prefix to indicate the number of atoms for the second element in the formula. 4. Use the root of the second elements name and the suffix -ide.

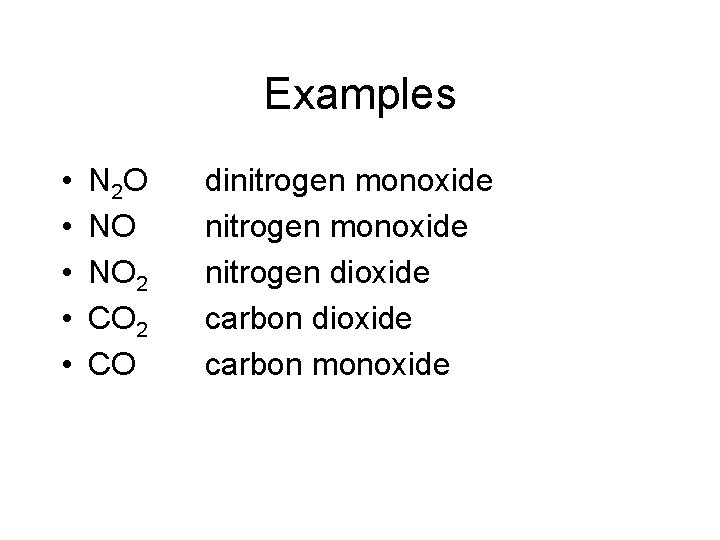
Examples • • • N 2 O NO NO 2 CO dinitrogen monoxide nitrogen dioxide carbon monoxide

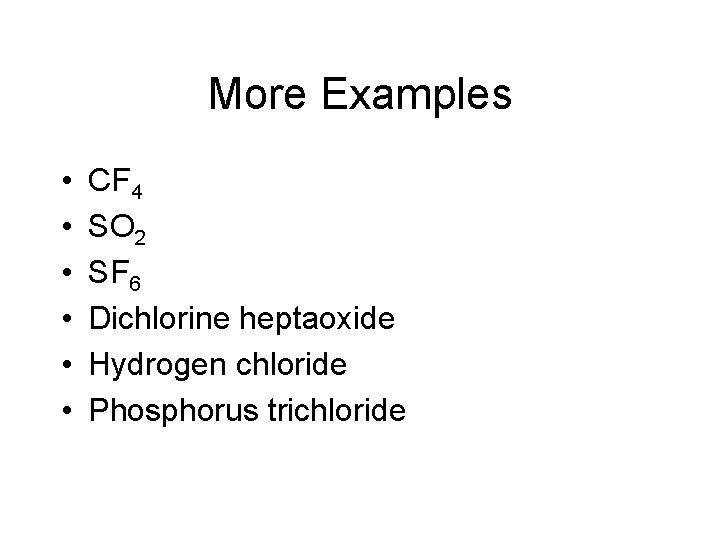
More Examples • • • CF 4 SO 2 SF 6 Dichlorine heptaoxide Hydrogen chloride Phosphorus trichloride

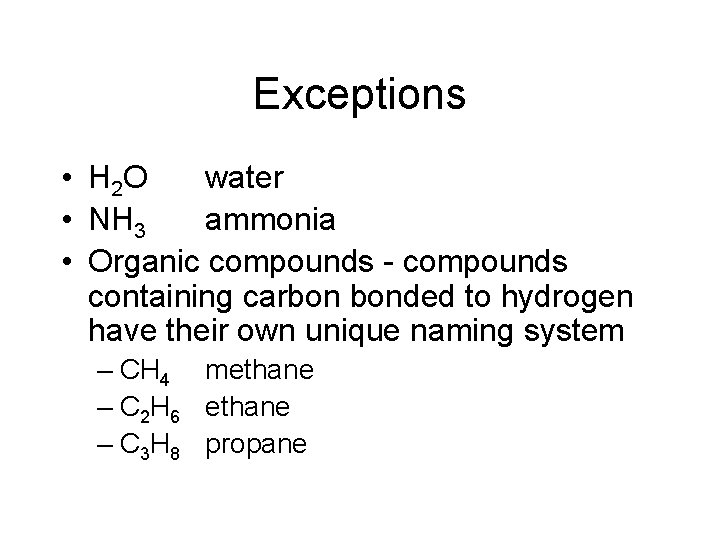
Exceptions • H 2 O water • NH 3 ammonia • Organic compounds - compounds containing carbon bonded to hydrogen have their own unique naming system – CH 4 methane – C 2 H 6 ethane – C 3 H 8 propane

The Stock Naming System The International Union of Pure and Applied Chemists (IUPAC) have developed a naming system based on the oxidation numbers of the first element in the formula. 1. Name the first element. 2. Use a Roman numeral to indicate the oxidation number, by reversing the criss-cross and determining the charge as if it were an ionic compound. 3. Use the root of the second elements name and end with the suffix -ide.

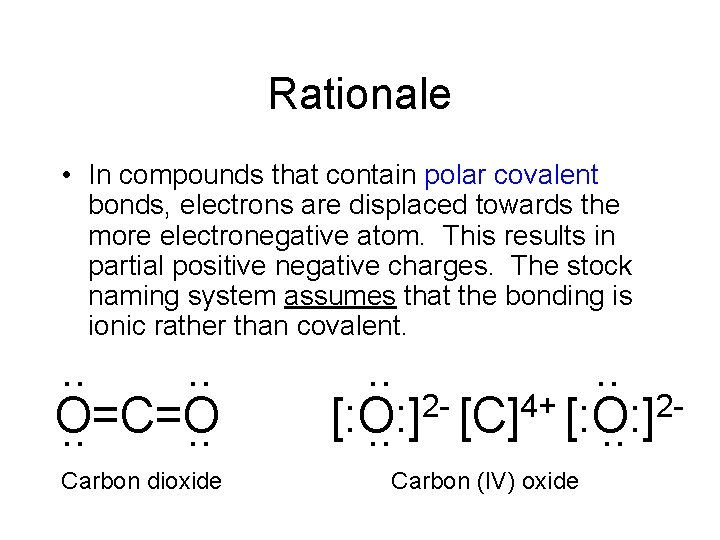
Rationale • In compounds that contain polar covalent bonds, electrons are displaced towards the more electronegative atom. This results in partial positive negative charges. The stock naming system assumes that the bonding is ionic rather than covalent. . . O=C=O. . Carbon dioxide . . 2 - [C]4+ [: O: ]2[: O: ]. . Carbon (IV) oxide

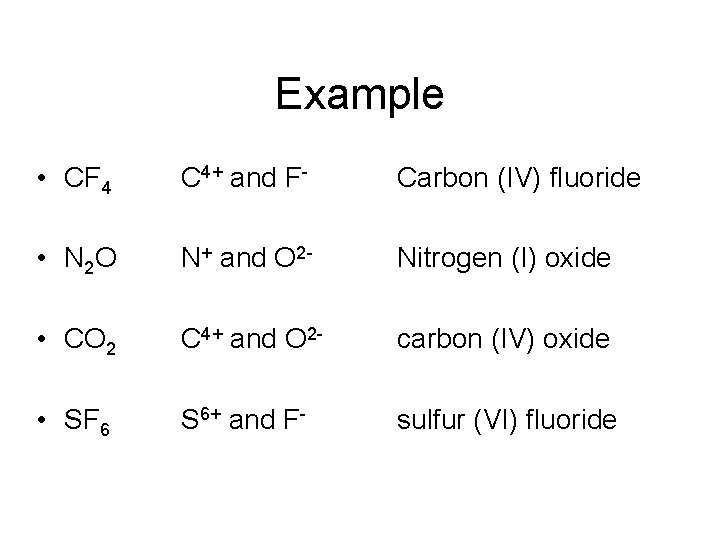
Example • CF 4 C 4+ and F- Carbon (IV) fluoride • N 2 O N+ and O 2 - Nitrogen (I) oxide • CO 2 C 4+ and O 2 - carbon (IV) oxide • SF 6 S 6+ and F- sulfur (VI) fluoride

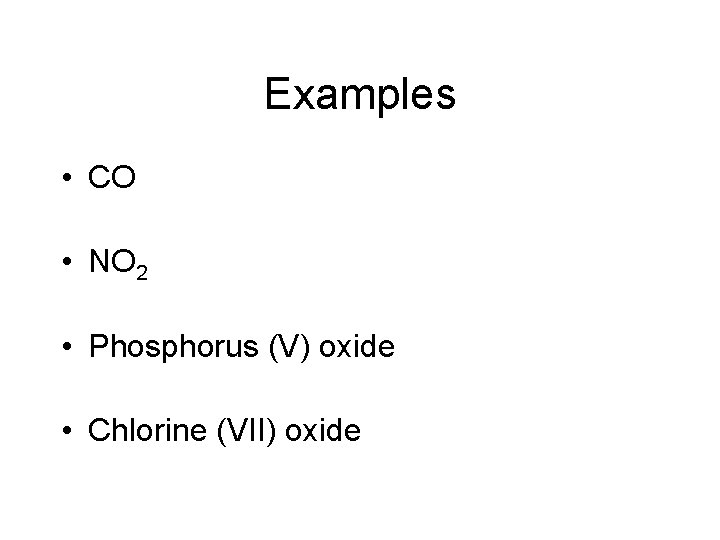
Examples • CO • NO 2 • Phosphorus (V) oxide • Chlorine (VII) oxide

Summary • Prefixes are used to indicate the number of each element in the formula • Stock naming system uses roman numerals to indicate the charge of the first element as determine from the criss -cross system.