Unit 61 Engineering Thermodynamics Lesson 11 Heat Engine


















- Slides: 56

Unit 61: Engineering Thermodynamics Lesson 11: Heat Engine Cycles

Objective • The purpose of this lesson is to examine a number of Heat Engine Cycles.

The Otto Cycle • The Otto cycle is the ideal air standard cycle for the spark-ignition piston engine. • In this cycle it is assumed that the working fluid, air, behaves as a perfect gas and that there is no change in the composition of the air during the complete cycle. • Heat transfer occurs at constant volume and there is isentropic compression and expansion

The Otto Cycle • Thus the Otto cycle is the ideal air standard cycle upon which the efficiency of the sparkignition piston engine is based.

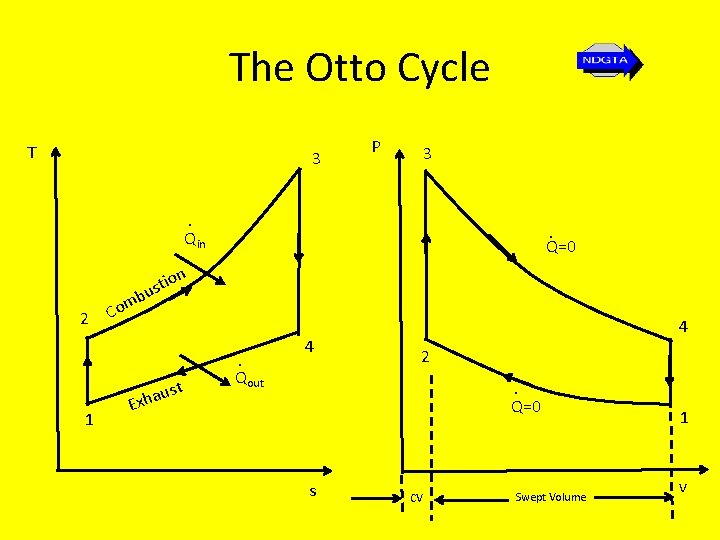
The Otto Cycle T 3 P 3 . Qin . Q=0 n io ust 2 1 mb o C st u a h Ex . Qout 4 4 2. Q=0 s CV Swept Volume 1 v

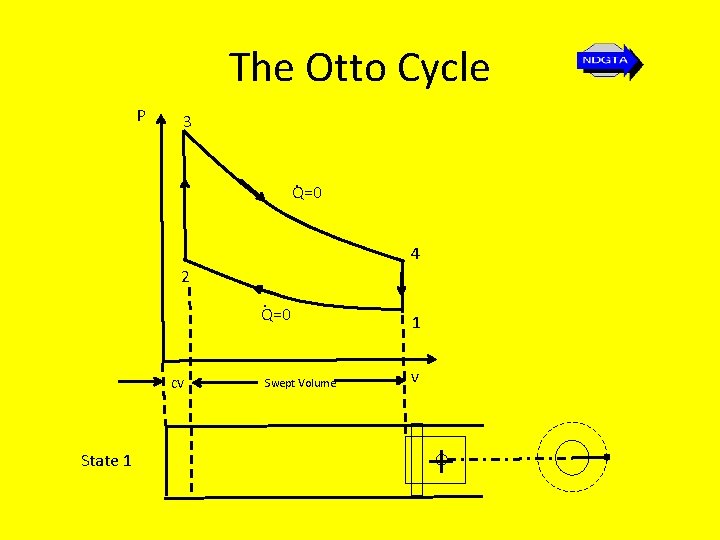
The Otto Cycle P 3. Q=0 4 2. Q=0 CV State 1 Swept Volume 1 v

The Otto Cycle State 1 State 2 State 3 State 4 State 1

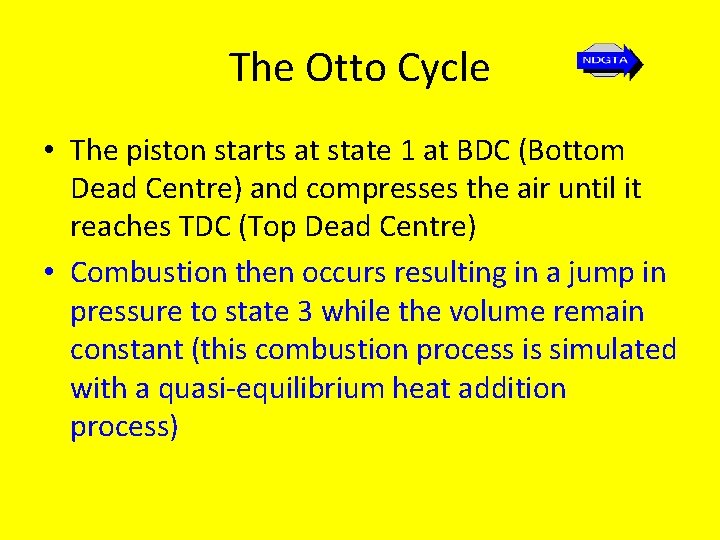
The Otto Cycle • 1 – 2: Isentropic compression - no heat transfer takes place, temperature and pressure increase and the volume decreases to the clearance volume • 2 – 3: Reversible constant volume heating temperature and pressure increase. • 3 – 4: Isentropic expansion (through swept volume) – air expands and does the work on the piston; pressure and temperature fall; no heat transfer takes place during the process • 4 -1: Reversible constant volume heat rejection (cooling) – pressure and temperature fall to original values

The Otto Cycle • The piston starts at state 1 at BDC (Bottom Dead Centre) and compresses the air until it reaches TDC (Top Dead Centre) • Combustion then occurs resulting in a jump in pressure to state 3 while the volume remain constant (this combustion process is simulated with a quasi-equilibrium heat addition process)

The Otto Cycle • The process that follows is the power stroke as the air (simulating the combustion products) expands isentropically to state 4. • In the final process heat transfer to the surroundings occurs and the cycle is completed. • The spark-ignition engine is modeled with this Otto cycle • Because the Otto cycle is the air standard cycle for the reciprocating piston internal combustion engine, its efficiency is known as the air standard thermal efficiency, ηair standard

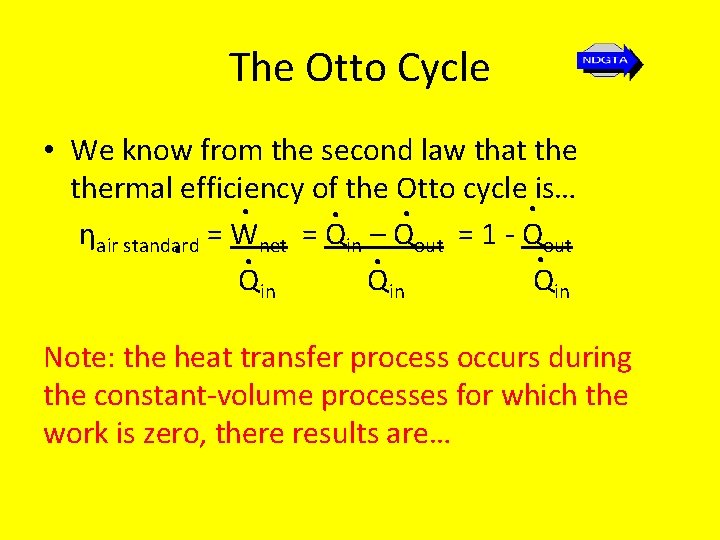
The Otto Cycle • We know from the second law that thermal efficiency of the Otto cycle is…. . ηair standard. = W. net = Qin –. Qout = 1 - Q. out Qin Qin Note: the heat transfer process occurs during the constant-volume processes for which the work is zero, there results are…

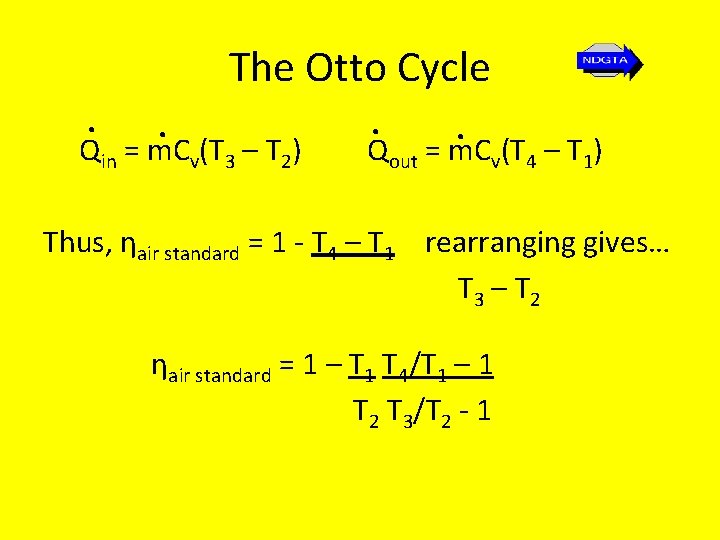
The Otto Cycle. . Q = m. C (T – T ) Q = m. C (T in v 3 2 out v 4 – T 1) Thus, ηair standard = 1 - T 4 – T 1 rearranging gives… T 3 – T 2 ηair standard = 1 – T 1 T 4/T 1 – 1 T 2 T 3/T 2 - 1

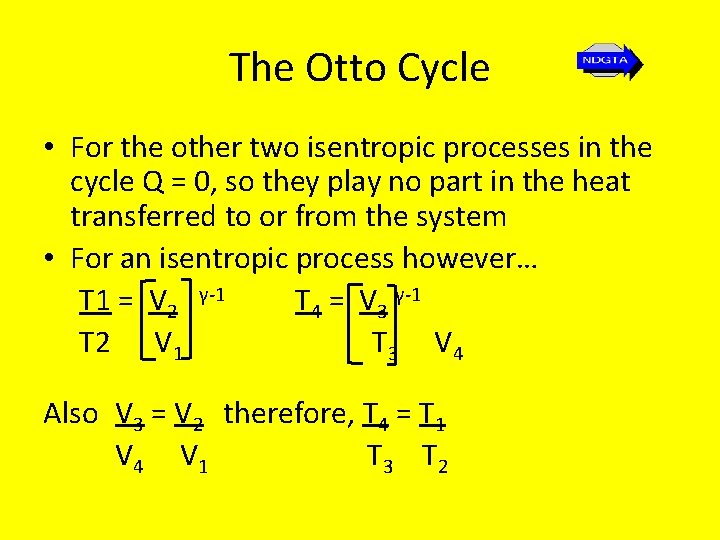
The Otto Cycle • For the other two isentropic processes in the cycle Q = 0, so they play no part in the heat transferred to or from the system • For an isentropic process however… T 1 = V 2 γ-1 T 4 = V 3 γ-1 T 2 V 1 T 3 V 4 Also V 3 = V 2 therefore, T 4 = T 1 V 4 V 1 T 3 T 2

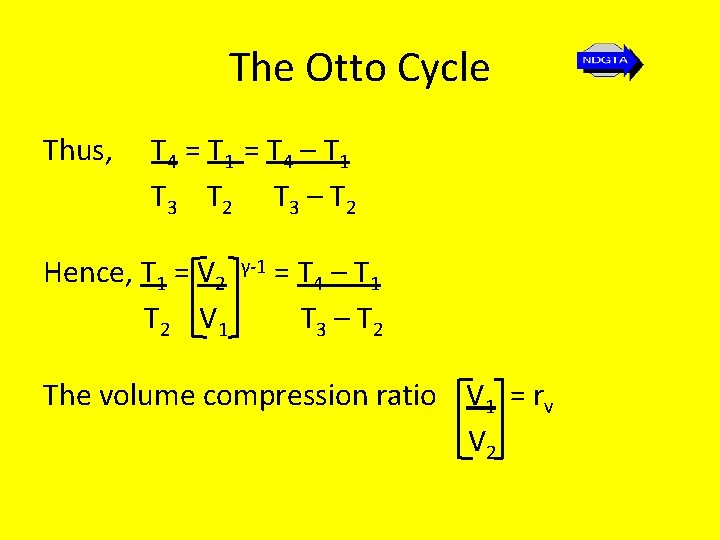
The Otto Cycle Thus, T 4 = T 1 = T 4 – T 1 T 3 T 2 T 3 – T 2 Hence, T 1 = V 2 T 2 V 1 γ-1 = T 4 – T 1 T 3 – T 2 The volume compression ratio V 1 = rv V 2

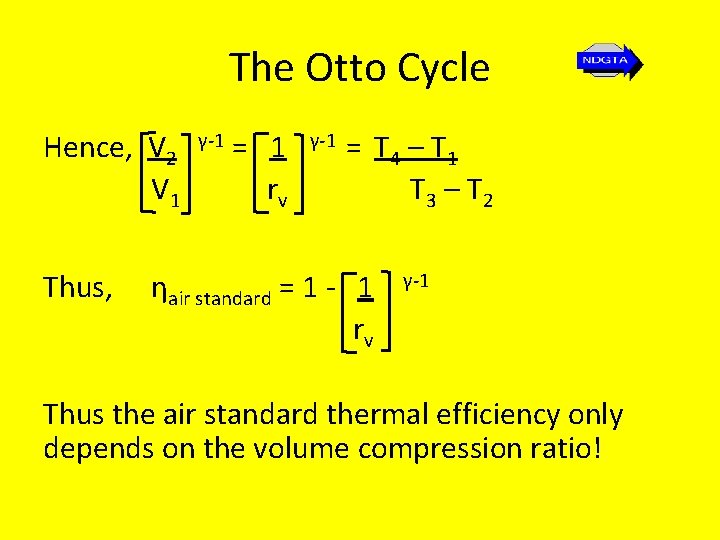
The Otto Cycle Hence, V 2 V 1 Thus, γ-1 = 1 rv γ-1 = T 4 – T 1 T 3 – T 2 ηair standard = 1 - 1 rv γ-1 Thus the air standard thermal efficiency only depends on the volume compression ratio!

The Otto Cycle • Calculate the Otto cycle air standard thermal efficiency for a reciprocating piston engine that has a swept volume of 210 cm 3 and a clearance volume (CV) of 30 cm 3. Total volume = swept volume + clearance Volume Thus volume compression ratio… rv = Total volume = 210 + 30 = 8 or 8: 1 Clearance Volume 30

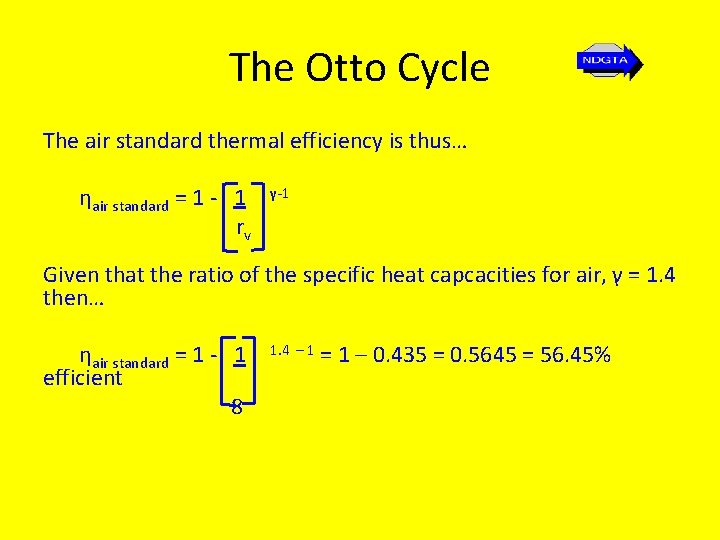
The Otto Cycle The air standard thermal efficiency is thus… ηair standard = 1 - 1 rv γ-1 Given that the ratio of the specific heat capcacities for air, γ = 1. 4 then… ηair standard = 1 - 1 efficient 8 1. 4 – 1 = 1 – 0. 435 = 0. 5645 = 56. 45%

The Reciprocating Piston Internal Combustion Engine • The reciprocating engine works on the four stroke or two stroke cycle. • In the spark-ignition petrol engine the power is generated by the fuel/air mixture being drawn into the cylinder during a suction stroke and being ignited by means of a spark towards the end of the compression stroke.

The Reciprocating Piston Internal Combustion Engine • In the case of the compression oil or diesel engine air only is drawn in during the suction stroke and towards the end of the compression stroke diesel fuel is injected • The high temperature of the compressed air induces the ignition of the fuel / air mix.

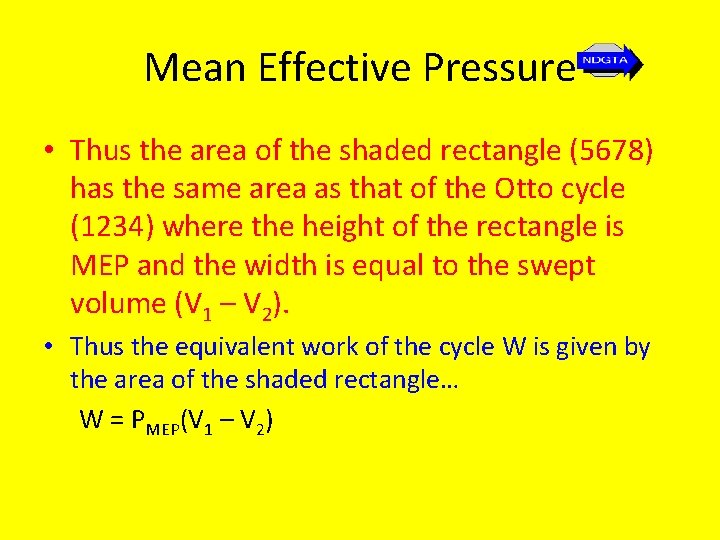
Mean Effective Pressure • When considering reciprocating piston combustion engines (as opposed to fixed plant), whether petrol or diesel powered, the most effective measure for comparing the performance of these engines is provided by finding their mean effective pressure, where… Mean effective pressure (MEP) = net work output swept volume

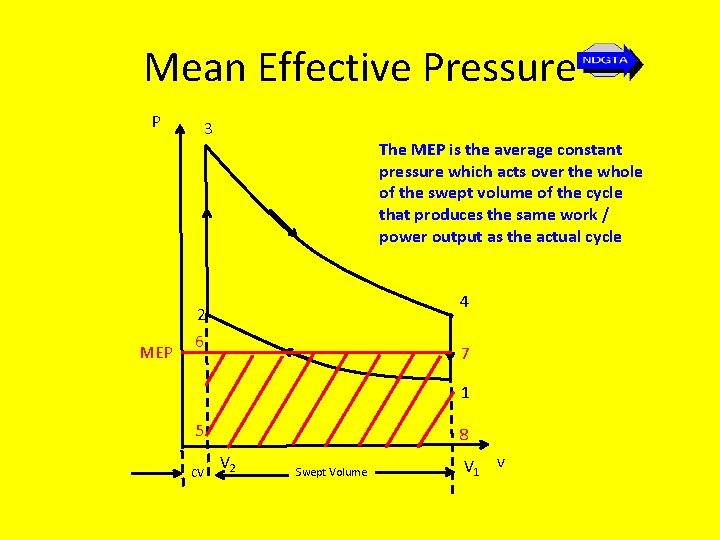
Mean Effective Pressure • The MEP is effectively a measure of engine work and hence power and is the average constant pressure that is assumed to act over the whole swept volume of the cycle.

Mean Effective Pressure P MEP 3 The MEP is the average constant pressure which acts over the whole of the swept volume of the cycle that produces the same work / power output as the actual cycle 4 2 6 7 1 5 CV 8 V 2 Swept Volume V 1 v

Mean Effective Pressure • Thus the area of the shaded rectangle (5678) has the same area as that of the Otto cycle (1234) where the height of the rectangle is MEP and the width is equal to the swept volume (V 1 – V 2). • Thus the equivalent work of the cycle W is given by the area of the shaded rectangle… W = PMEP(V 1 – V 2)

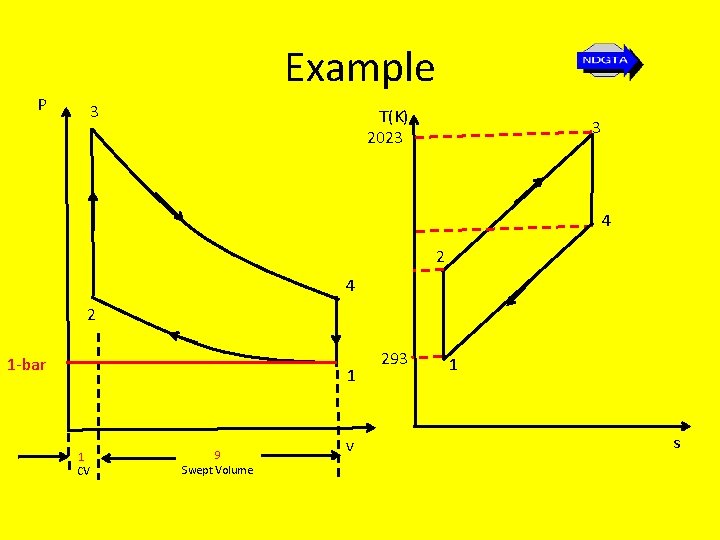
Example • In an Otto cycle at the start of the compression the air is at a pressure of 1 -bar and a temperature of 20 o. C. If the volume compression ratio is 9 and the maximum cycle temperature is 1750 o. C, determine thermal efficiency, the net work and the MEP

Example P 3 T(K) 2023 3 4 2 1 -bar 1 1 CV 9 Swept Volume v 293 1 s

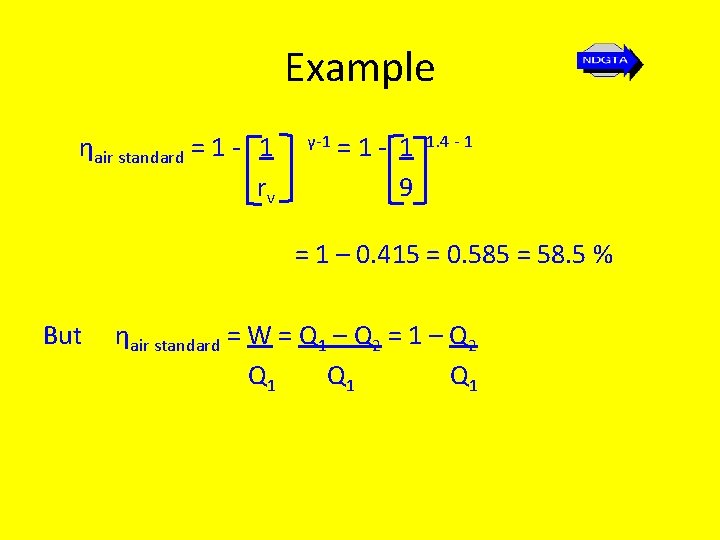
Example ηair standard = 1 - 1 rv γ-1 =1 - 1 9 1. 4 - 1 = 1 – 0. 415 = 0. 585 = 58. 5 % But ηair standard = W = Q 1 – Q 2 = 1 – Q 2 Q 1 Q 1

Example • Also the heat addition Q 1 = c. V(T 3 – T 2), hence ηair standard = Wnet Q 1 c. V(T 3 – T 2) Thus Wnet = ηair standard c. V(T 3 – T 2)

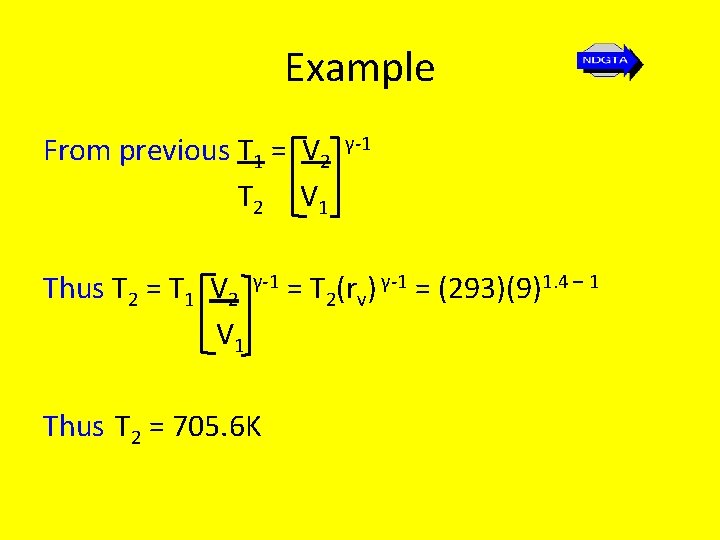
Example From previous T 1 = V 2 T 2 V 1 γ-1 Thus T 2 = T 1 V 2 γ-1 = T 2(rv) γ-1 = (293)(9)1. 4 – 1 V 1 Thus T 2 = 705. 6 K

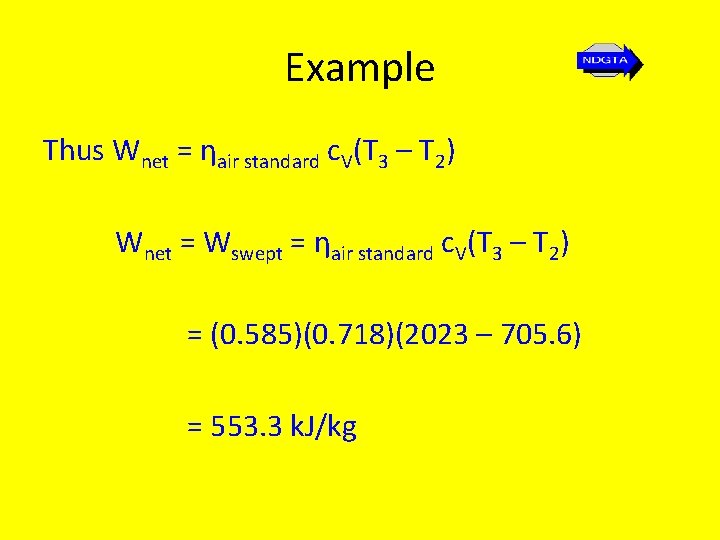
Example Thus Wnet = ηair standard c. V(T 3 – T 2) Wnet = Wswept = ηair standard c. V(T 3 – T 2) = (0. 585)(0. 718)(2023 – 705. 6) = 553. 3 k. J/kg

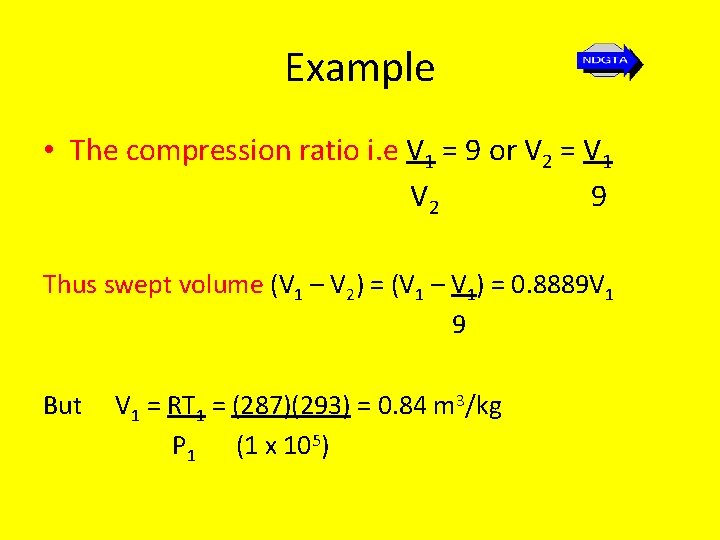
Example • In order to find the MEP we first need to find the swept volume (V 1 – V 2). • Since the working fluid is air, we can use the gas equation for unit mass… p 1 V 1 = RT 1 rearranging gives V 1 = RT 1 P 1

Example • The compression ratio i. e V 1 = 9 or V 2 = V 1 V 2 9 Thus swept volume (V 1 – V 2) = (V 1 – V 1) = 0. 8889 V 1 9 But V 1 = RT 1 = (287)(293) = 0. 84 m 3/kg P 1 (1 x 105)

Example So swept volume (V 1 – V 2) = (0. 8889)(0. 84) = 0. 747 k. J/kg So knowing the swept volume and the net work (or swept work) we can now find the MEP… MEP = 553 = 740. 7 k. J/m 3 = 740. 7 k. N/m 2 = 740. 7 bar 0. 747

The Diesel Cycle • The original diesel engine had the fuel (powdered coal) forced in using compressed air. • The ideal air standard diesel cycle is the cycle that most closely models this original diesel engine. • Modern oil engines or compression engines with direct fuel injection are based on the dual combustion cycle

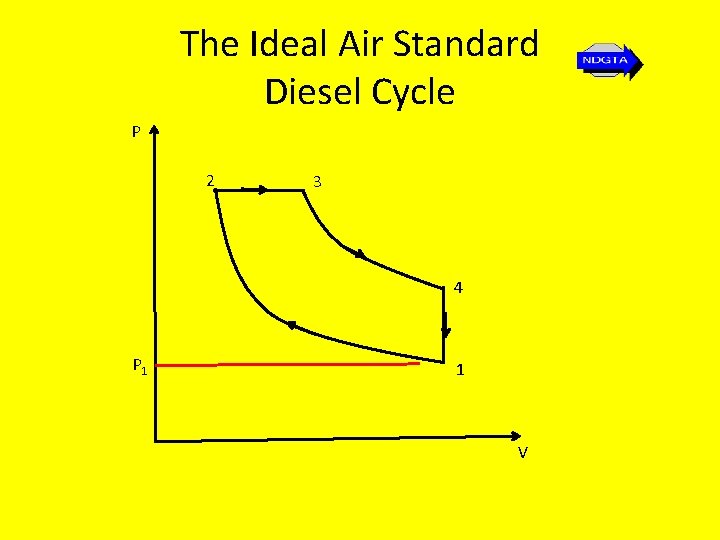
The Ideal Air Standard Diesel Cycle P 2 3 4 P 1 1 V

The Ideal Air Standard Diesel Cycle Stage 1 – 2: Stage 2 - 3: Stage 3 - 4: Stage 4 - 1: Isentropic compression Reversible constant pressure heat supply Isentropic expansion Reversible constant volume heat rejection

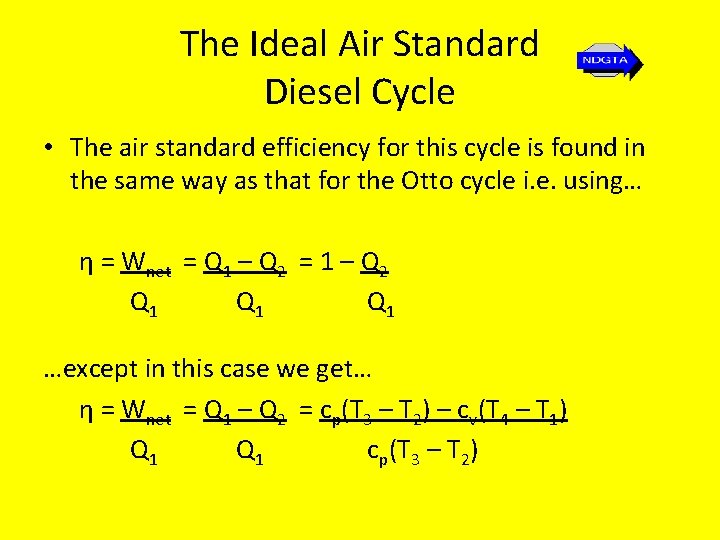
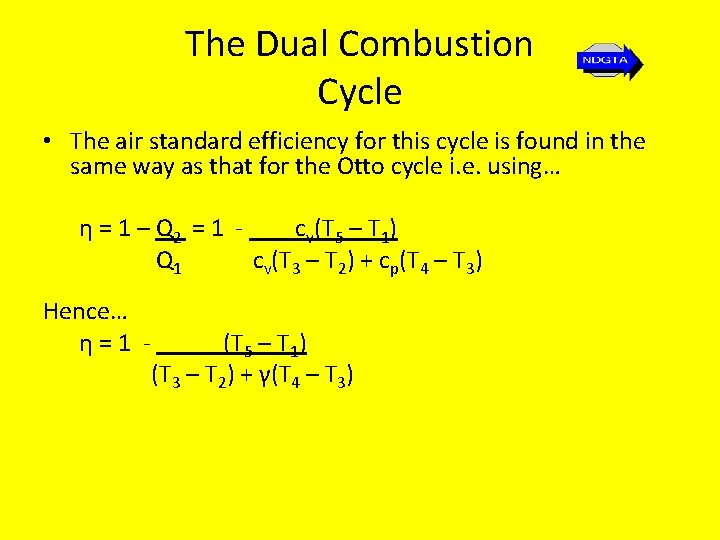
The Ideal Air Standard Diesel Cycle • The air standard efficiency for this cycle is found in the same way as that for the Otto cycle i. e. using… η = Wnet = Q 1 – Q 2 = 1 – Q 2 Q 1 Q 1 …except in this case we get… η = Wnet = Q 1 – Q 2 = cp(T 3 – T 2) – cv(T 4 – T 1) Q 1 cp(T 3 – T 2)

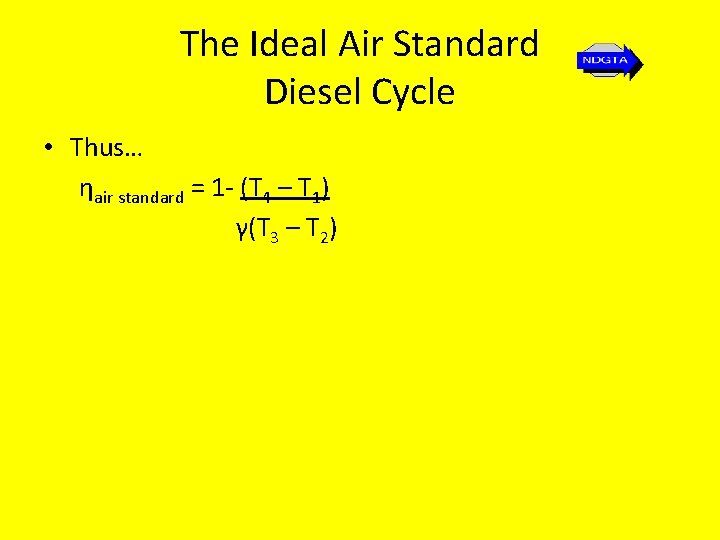
The Ideal Air Standard Diesel Cycle • Thus… ηair standard = 1 - (T 4 – T 1) γ(T 3 – T 2)

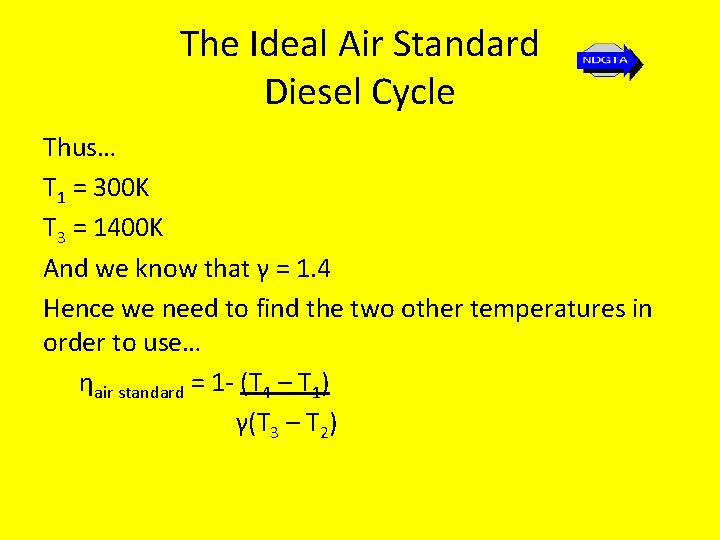
The Ideal Air Standard Diesel Cycle • A diesel engine with a compression ratio of 15: 1 operates with an inlet pressure of 1 -bar and an inlet temperature of 300 K. If the maximum cycle temperature is 1400 K, determine the air standard thermal efficiency based on the diesel cycle.

The Ideal Air Standard Diesel Cycle Thus… T 1 = 300 K T 3 = 1400 K And we know that γ = 1. 4 Hence we need to find the two other temperatures in order to use… ηair standard = 1 - (T 4 – T 1) γ(T 3 – T 2)

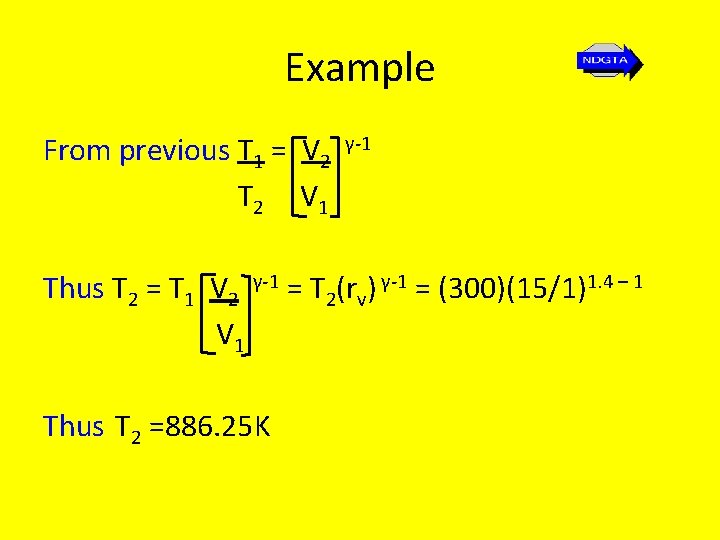
Example From previous T 1 = V 2 T 2 V 1 γ-1 Thus T 2 = T 1 V 2 γ-1 = T 2(rv) γ-1 = (300)(15/1)1. 4 – 1 V 1 Thus T 2 =886. 25 K

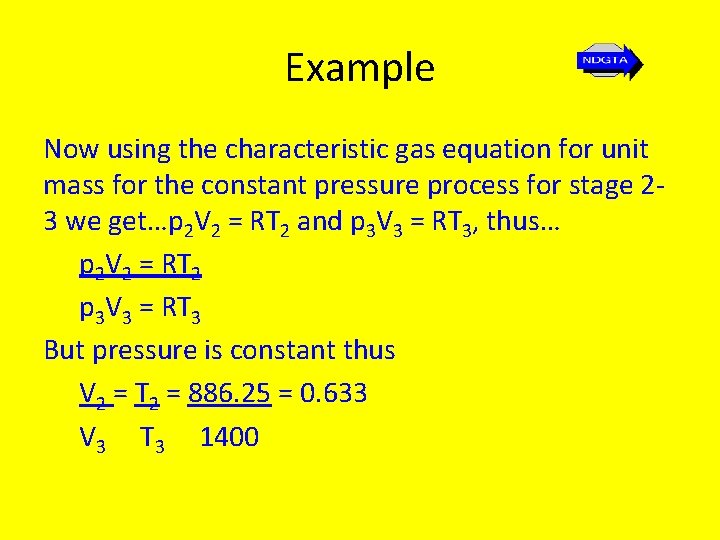
Example Now using the characteristic gas equation for unit mass for the constant pressure process for stage 23 we get…p 2 V 2 = RT 2 and p 3 V 3 = RT 3, thus… p 2 V 2 = RT 2 p 3 V 3 = RT 3 But pressure is constant thus V 2 = T 2 = 886. 25 = 0. 633 V 3 T 3 1400

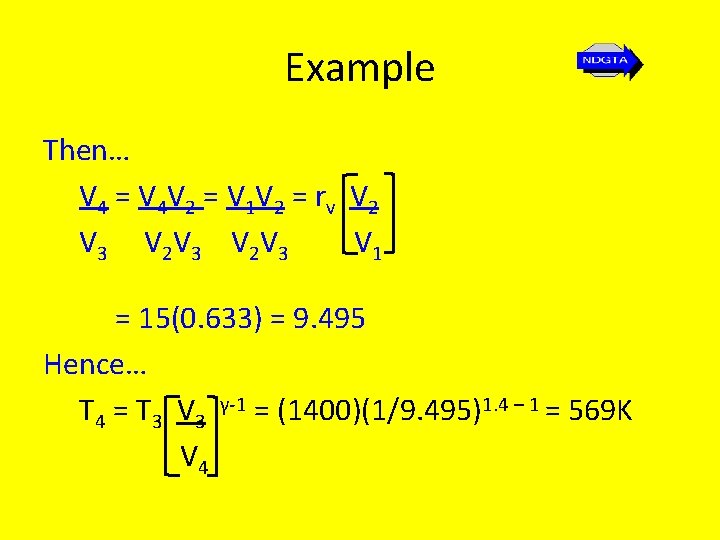
Example Then… V 4 = V 4 V 2 = V 1 V 2 = r v V 2 V 3 V 1 = 15(0. 633) = 9. 495 Hence… T 4 = T 3 V 3 γ-1 = (1400)(1/9. 495)1. 4 – 1 = 569 K V 4

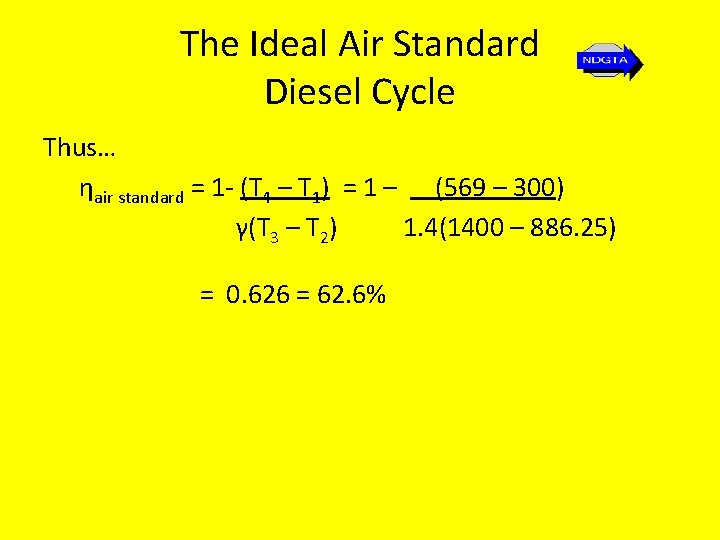
The Ideal Air Standard Diesel Cycle Thus… ηair standard = 1 - (T 4 – T 1) = 1 – (569 – 300) γ(T 3 – T 2) 1. 4(1400 – 886. 25) = 0. 626 = 62. 6%

The Dual Combustion Cycle • As mentioned previously the dual combustion cycle is the cycle on which the modern compression-ignition oil engine operating cycle is based. • In this cycle the heat is supplied to the system from the fuel is provided partly at constant volume and partly at constant pressure

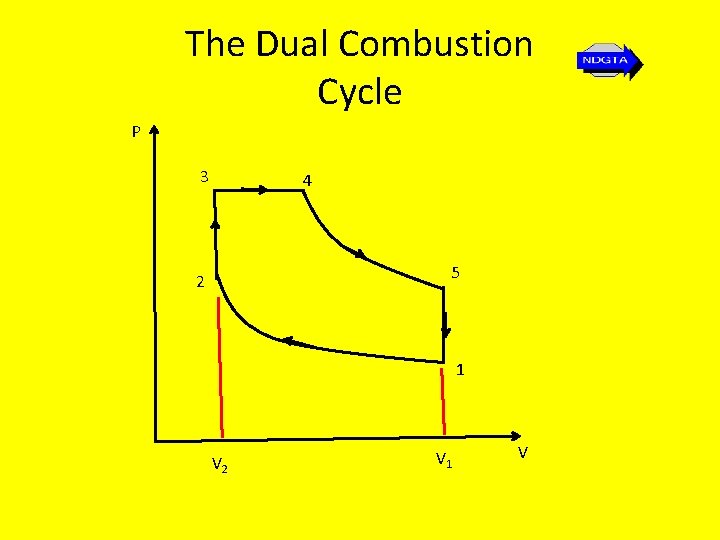
The Dual Combustion Cycle P 3 4 5 2 1 V 2 V 1 V

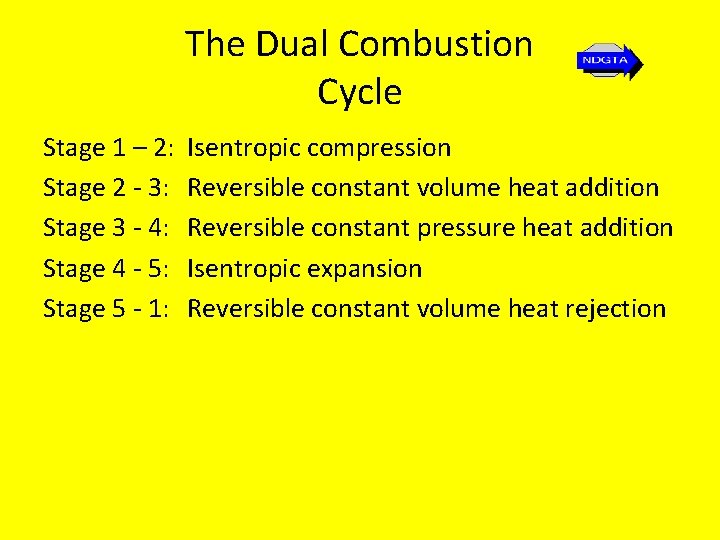
The Dual Combustion Cycle Stage 1 – 2: Stage 2 - 3: Stage 3 - 4: Stage 4 - 5: Stage 5 - 1: Isentropic compression Reversible constant volume heat addition Reversible constant pressure heat addition Isentropic expansion Reversible constant volume heat rejection

The Dual Combustion Cycle • As can be seen from the stages of this cycle the heat from the fuel is supplied in two stages. • In the first stage combustion takes place at constant volume • In the second stage combustion takes place at at constant pressure

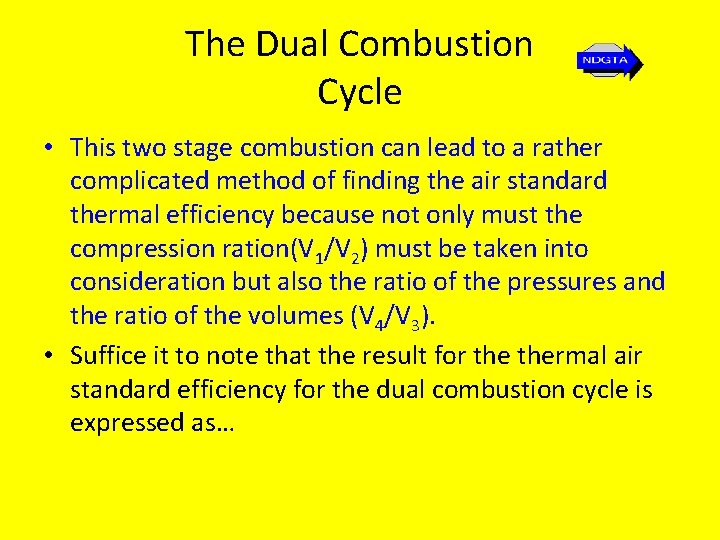
The Dual Combustion Cycle • This two stage combustion can lead to a rather complicated method of finding the air standard thermal efficiency because not only must the compression ration(V 1/V 2) must be taken into consideration but also the ratio of the pressures and the ratio of the volumes (V 4/V 3). • Suffice it to note that the result for thermal air standard efficiency for the dual combustion cycle is expressed as…

The Dual Combustion Cycle • The air standard efficiency for this cycle is found in the same way as that for the Otto cycle i. e. using… η = 1 – Q 2 = 1 cv(T 5 – T 1) Q 1 cv(T 3 – T 2) + cp(T 4 – T 3) Hence… η=1 - (T 5 – T 1) (T 3 – T 2) + γ(T 4 – T 3)

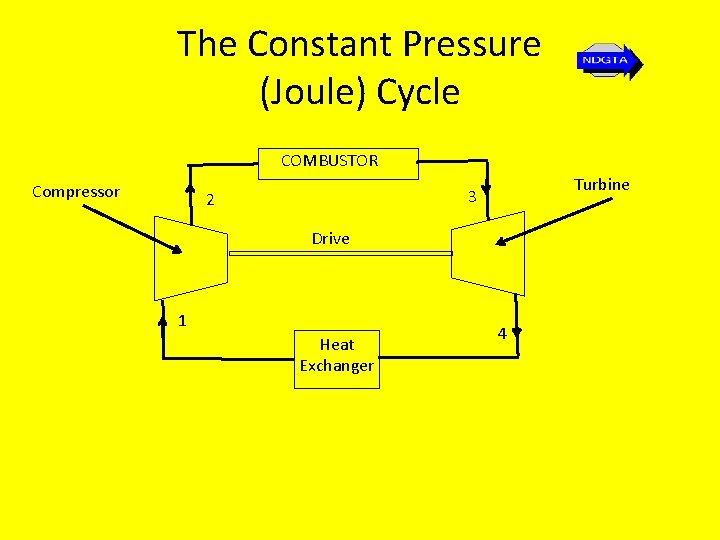
The Constant Pressure (Joule) Cycle • This is the ideal cycle for a closed system gas turbine plant • A typical gas turbine plant layout is shown below…

The Constant Pressure (Joule) Cycle COMBUSTOR Compressor Turbine 3 2 Drive 1 Heat Exchanger 4

The Constant Pressure (Joule) Cycle • Such a plant consists typically of a compressor, where the air is subject to isentropic compression, a combustor, where heat is added at constant pressure, a turbine, where air is subject to isentropic expansion and a heat exchanger where the heat is rejected (extracted) again at constant pressure

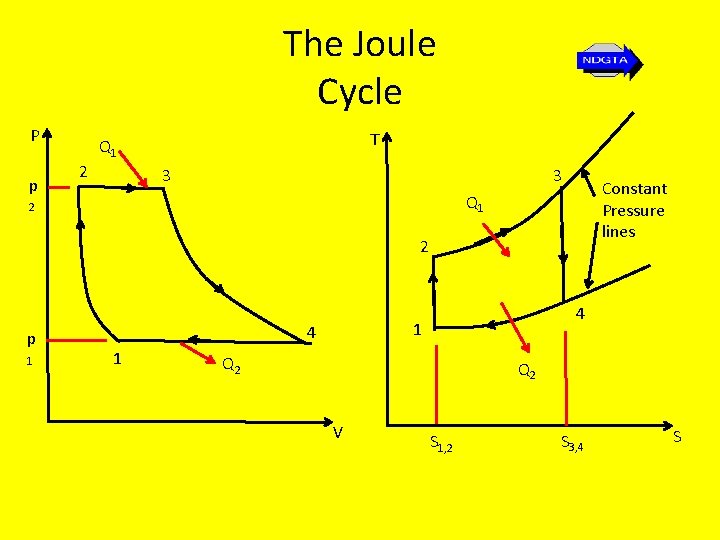
The Constant Pressure (Joule) Cycle Stage 1 – 2: Stage 2 - 3: Stage 3 - 4: Stage 4 - 1: Isentropic compression Reversible constant pressure heat supply Isentropic expansion Reversible constant pressure heat rejection

The Joule Cycle P p 2 T Q 1 3 3 Constant Pressure lines Q 1 2 2 p 1 1 4 Q 2 V S 1, 2 S 3, 4 S

The Practical Four-stroke cycle • The sequence of operations by which the four stroke engine converts heat energy into mechanical energy is known as the four-stroke cycle. • A mixture of petrol and air is introduced into the cylinder during the induction stroke and compressed during the compression stroke. • At this point the fuel is ignited and the pressure wave produced by ignited fuel drives the piston down on its power stroke

The Practical Four-stroke cycle • Finally the waste products of combustion are ejected during the exhaust stroke.