Unit 6 Unit 6 Unit 6 Unit 6









- Slides: 27

Unit 6

Unit 6

Unit 6

Unit 6: Particles with Internal Structure The Role of Charge

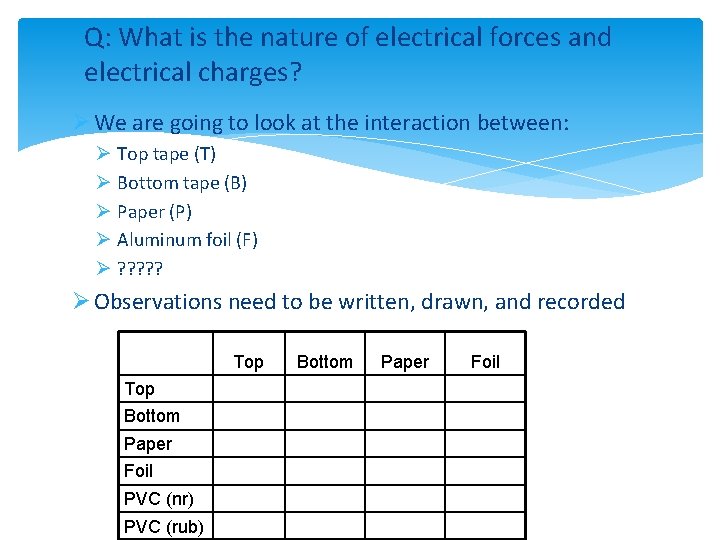
Q: What is the nature of electrical forces and electrical charges? Ø We are going to look at the interaction between: Ø Top tape (T) Ø Bottom tape (B) Ø Paper (P) Ø Aluminum foil (F) Ø ? ? ? Ø Observations need to be written, drawn, and recorded Top Bottom Paper Foil PVC (nr) PVC (rub) Bottom Paper Foil

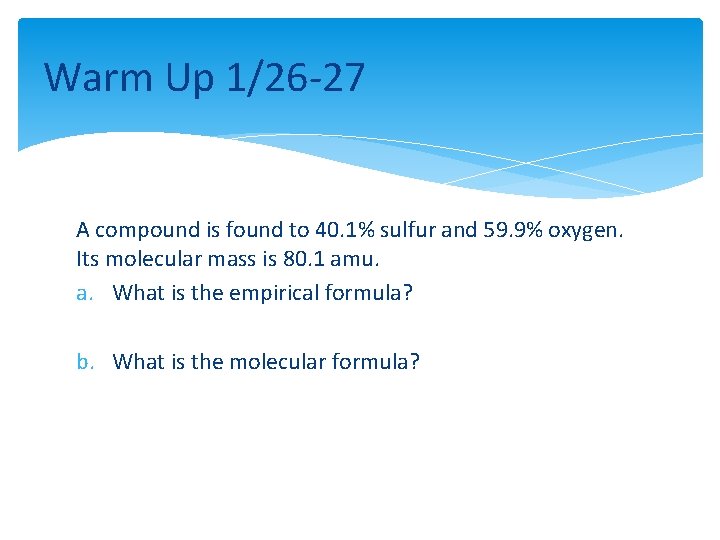
Warm Up 1/26 -27 A compound is found to 40. 1% sulfur and 59. 9% oxygen. Its molecular mass is 80. 1 amu. a. What is the empirical formula? b. What is the molecular formula?

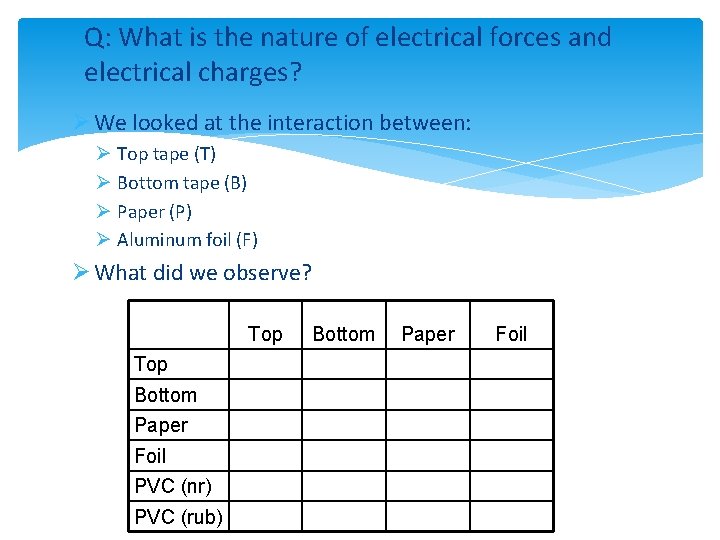
Q: What is the nature of electrical forces and electrical charges? Ø We looked at the interaction between: Ø Top tape (T) Ø Bottom tape (B) Ø Paper (P) Ø Aluminum foil (F) Ø What did we observe? Top Bottom Paper Foil PVC (nr) PVC (rub) Bottom Paper Foil

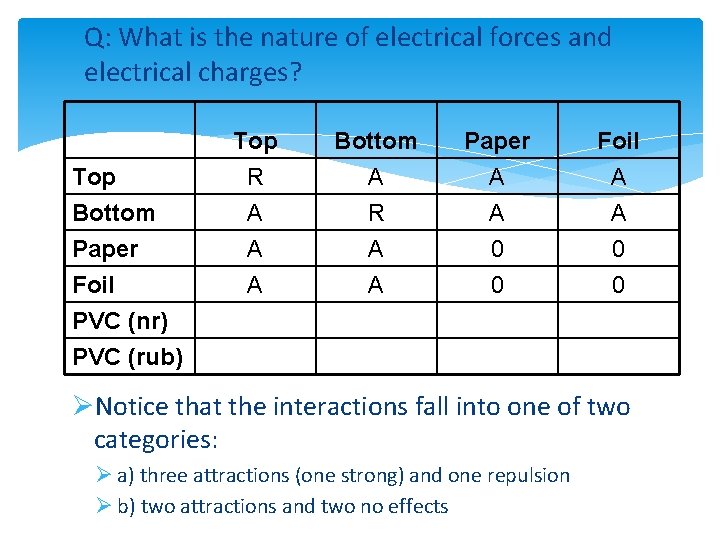
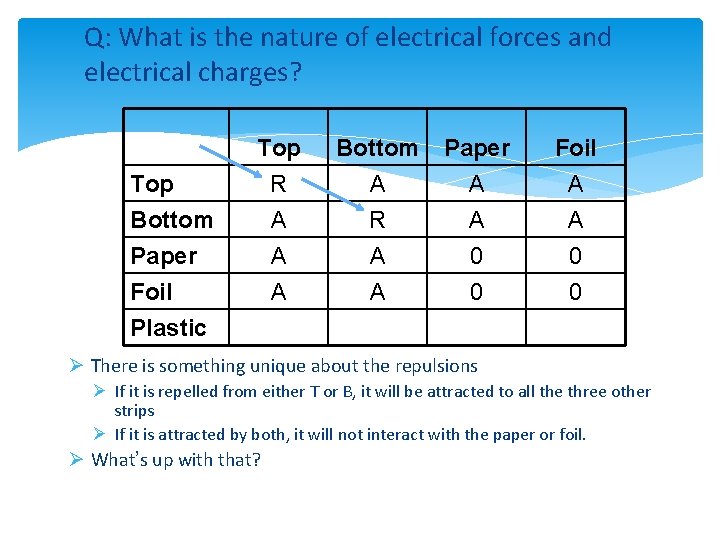
Q: What is the nature of electrical forces and electrical charges? Top Bottom Paper Foil PVC (nr) PVC (rub) Top R A A A Bottom A R A A Paper A A 0 0 Foil A A 0 0 ØNotice that the interactions fall into one of two categories: Ø a) three attractions (one strong) and one repulsion Ø b) two attractions and two no effects

Q: What is the nature of electrical forces and electrical charges? Top Bottom Paper Foil Plastic Top R A A A Bottom Paper A A R A A 0 Foil A A 0 0 Ø There is something unique about the repulsions Ø If it is repelled from either T or B, it will be attracted to all the three other strips Ø If it is attracted by both, it will not interact with the paper or foil. Ø What’s up with that?

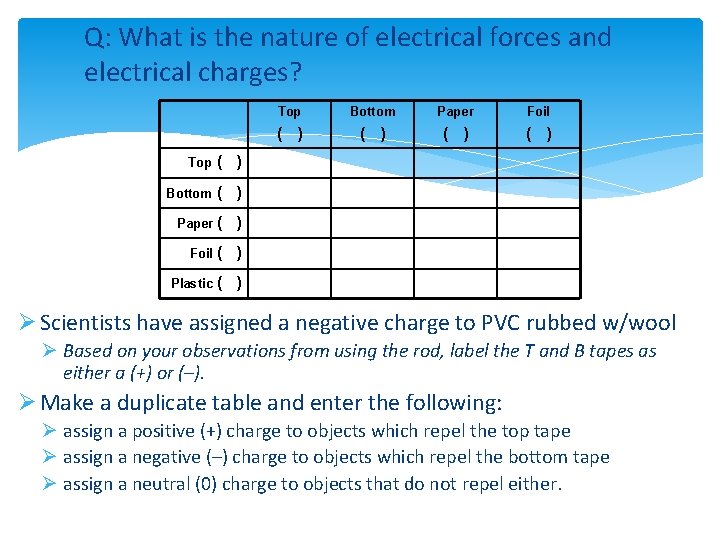
Q: What is the nature of electrical forces and electrical charges? Top ( ) Bottom ( ) Paper ( ) Foil ( ) Plastic ( ) Top Bottom Paper Foil ( ) ( ) Ø Scientists have assigned a negative charge to PVC rubbed w/wool Ø Based on your observations from using the rod, label the T and B tapes as either a (+) or (–). Ø Make a duplicate table and enter the following: Ø assign a positive (+) charge to objects which repel the top tape Ø assign a negative (–) charge to objects which repel the bottom tape Ø assign a neutral (0) charge to objects that do not repel either.

Q: What is the nature of electrical forces and electrical charges? ØHow can you be sure something has a net charge? ØWhat happened to the force of the interaction as the objects approached each other? ØDoes that fit with our particle model and Eph?

Model Summary Update Ø What’s new? Ø There is something smaller than the atom Ø Evidence of internal structure for our atoms Ø Particle with charge Ø Assume atoms contain both positive and negative charges that normally cancel each other. Ø HDYK that is the case? Ø We will use the Thomson model of the atom Ø a massive positive core Ø a small number of mobile, negatively charged particles we call “electrons”. (He called them corpuscles) Ø AKA: Plum pudding Model Ø Evidence led J. J. Thomson to propose that in solids Ø only the negative charges are free to move Ø these charges are much smaller than an atom and contribute a negligible fraction of its mass

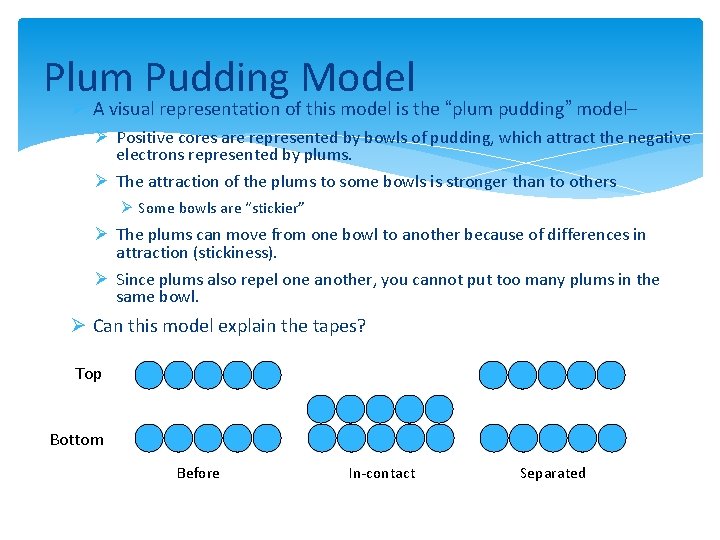
Plum Pudding Model Ø A visual representation of this model is the “plum pudding” model– Ø Positive cores are represented by bowls of pudding, which attract the negative electrons represented by plums. Ø The attraction of the plums to some bowls is stronger than to others Ø Some bowls are “stickier” Ø The plums can move from one bowl to another because of differences in attraction (stickiness). Ø Since plums also repel one another, you cannot put too many plums in the same bowl. Ø Can this model explain the tapes? Top Bottom Before In-contact Separated

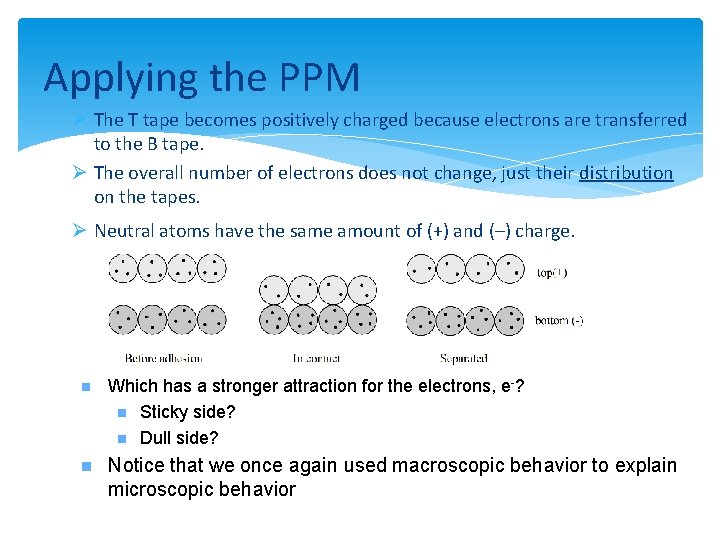
Applying the PPM Ø The T tape becomes positively charged because electrons are transferred to the B tape. Ø The overall number of electrons does not change, just their distribution on the tapes. Ø Neutral atoms have the same amount of (+) and (–) charge. n Which has a stronger attraction for the electrons, e-? n Sticky side? n Dull side? n Notice that we once again used macroscopic behavior to explain microscopic behavior

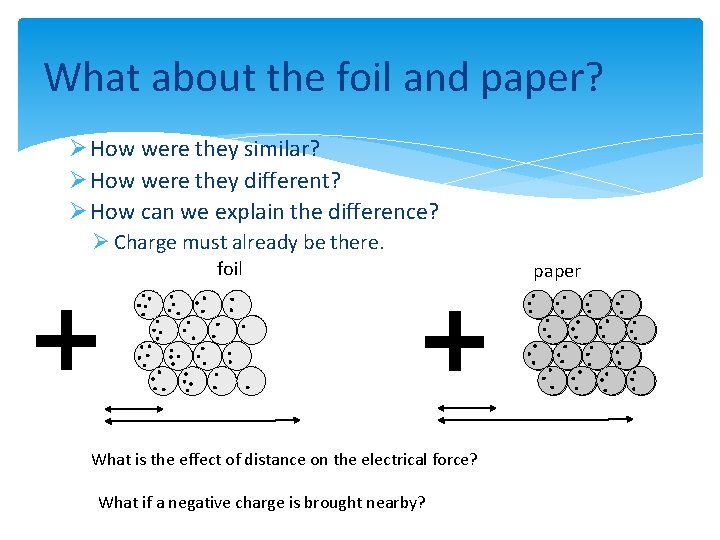
What about the foil and paper? Ø How were they similar? Ø How were they different? Ø How can we explain the difference? Ø Charge must already be there. foil What is the effect of distance on the electrical force? What if a negative charge is brought nearby? paper

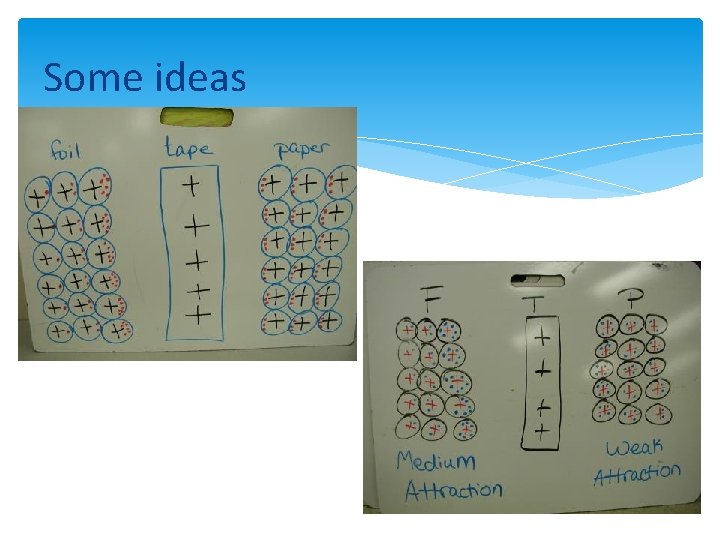
Some ideas

Examples Ø https: //phet. colorado. edu Ø 1) Balloons and Static Electricity Ø 2) John Travoltage

Warm Up 2/1 Ø In the sticky tape lab, which side has a stronger attraction for the electrons—sticky or dull? Ø Explain how you know (What evidence do you have? )

JJ Thomson’s BIG Idea Ø He observed a cathode ray tube and noticed that he could change the path of the “corpuscles” with a magnet Ø The direction of the deflection indicated a negative charge for the corpuscles Ø What were Thomson’s corpuscles? Ø electrons cathode glass tube anode

How do we apply this model to compounds? Ø We can propose that electrical forces are involved in holding together the particles that make up pure substances. Ø Perhaps the mobile negative charge is freer to move in some substances than in others. Ø Foil vs. paper Ø Metals and non-metals Ø Conductors and insulators

Overview of the Modern Periodic Table ØTwo distinct regions of note: ØMetals (M) ØGood conductor of electricity Ø 2/3 of table are metals ØIncludes transition elements (Groups 3 -12) ØNon-metals (NM) ØPoor conductors of electricity ØAll of groups 17 & 18; some of groups 14 -16 ØExhibit a wide range of properties

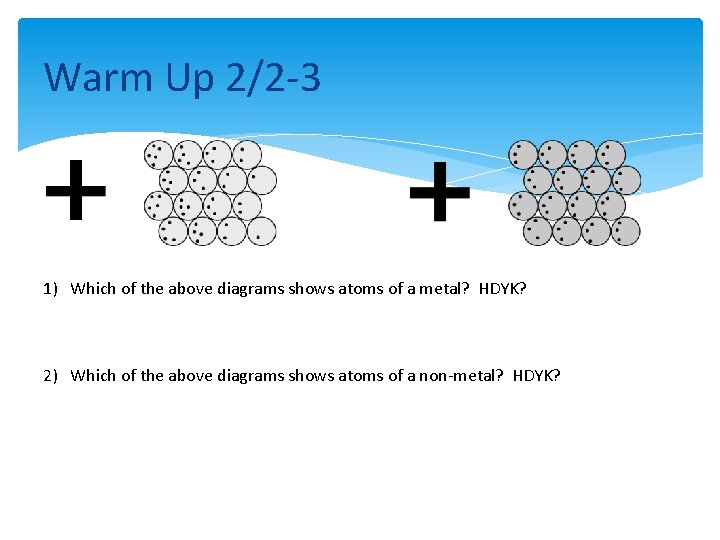
Warm Up 2/2 -3 1) Which of the above diagrams shows atoms of a metal? HDYK? 2) Which of the above diagrams shows atoms of a non-metal? HDYK?

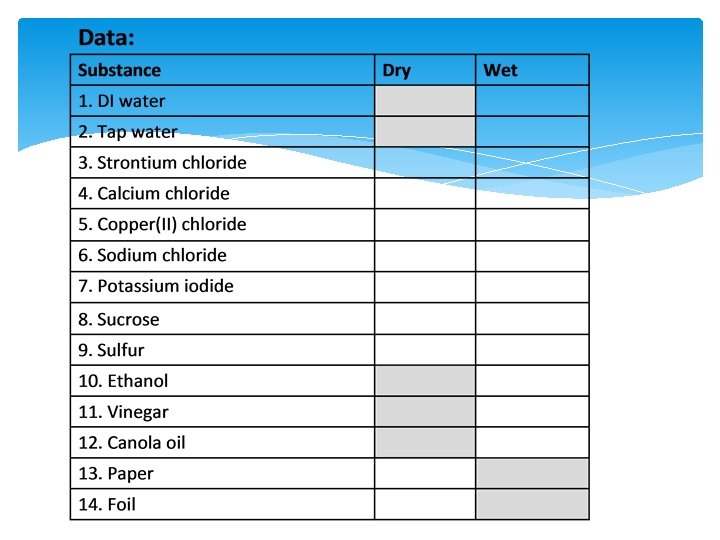
Lab 6. 2: Conductivity of substances and solutions



Assignment Ø Analyze your Lab 6. 2 Data Ø Look for patterns Ø Position on the periodic table Ø Metals/Non-metals Ø Write a conclusion in your journal Ø Claim Ø Evidence Ø Reasoning

Exit Ticket 2/2 -3 Ø 1) Based on your data from today’s lab, make a CLAIM. Ø 2) What EVIDENCE (data) do you have to support your claim? Ø 3) Support your conclusion with REASONING.