Unit 6 The Mole Composition and Emperical Formula







- Slides: 30

Unit 6 The Mole: % Composition and Emperical Formula PLEASE GRAB A CALCULATOR FROM THE BACK CHECK YOUR BOX

Formula Mass Molecular mass – the sum of the average atomic masses of all the atoms in a molecule ***Remember*** A molecule is a neutral group of atoms that are held together by covalent bonds!!!

The Mole and Molar Mass Ø The atomic molar mass is the mass of one mole of atoms. This is the value found on your Periodic Table. ü Ø All values are rounded to three decimal places. The term molar mass is a general term referring to the mass of one mole of a compound. Also called molecular mass, formula mass, molar mass. Ø Add the molar mass together for each atom bonded together

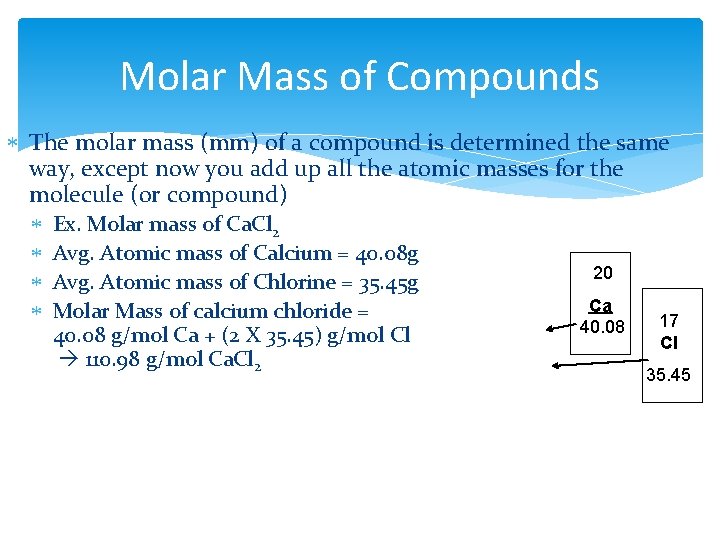
Molar Mass of Compounds The molar mass (mm) of a compound is determined the same way, except now you add up all the atomic masses for the molecule (or compound) Ex. Molar mass of Ca. Cl 2 Avg. Atomic mass of Calcium = 40. 08 g Avg. Atomic mass of Chlorine = 35. 45 g Molar Mass of calcium chloride = 40. 08 g/mol Ca + (2 X 35. 45) g/mol Cl 110. 98 g/mol Ca. Cl 2 20 Ca 40. 08 17 Cl 35. 45

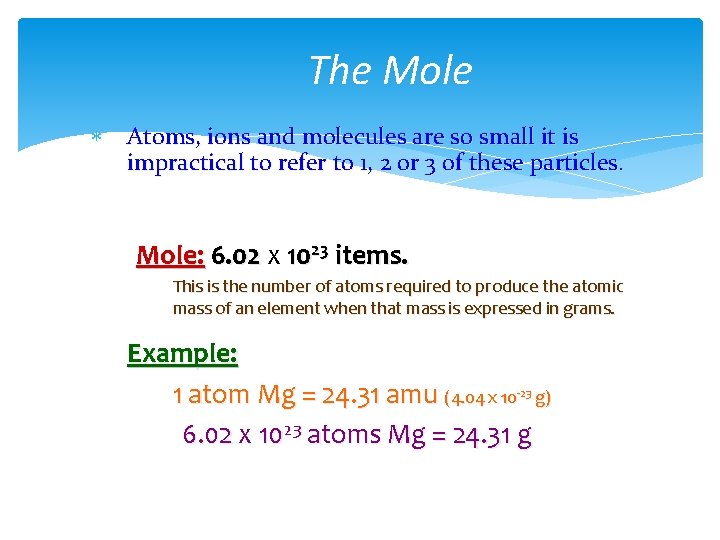
The Mole Atoms, ions and molecules are so small it is impractical to refer to 1, 2 or 3 of these particles. Mole: 6. 02 x 1023 items. This is the number of atoms required to produce the atomic mass of an element when that mass is expressed in grams. Example: 1 atom Mg = 24. 31 amu (4. 04 x 10 -23 g) 6. 02 x 1023 atoms Mg = 24. 31 g

Avogadro’s Number (symbol N) is the number of atoms in 12. 01 grams of carbon. Its numerical value is 6. 02 × 1023. Therefore, a 12. 01 g sample of carbon contains 6. 02 × 1023 carbon atoms.

The Mole Reading Balanced Equations 2 Mg(s) + O 2(g) 2 Mg. O(s) Ø Ø 2 Mg atoms + 1 O 2 molecule produces 2 Mg. O formula units. 12. 04 x 1023 Mg atoms + 6. 02 x 1023 O 2 molecules produces 12. 04 x 1023 Mg. O formula units. 2 mol Mg atoms + 1 mol O 2 molecules produces 2 mol Mg. O formula units. The terms atoms, molecules and formula units are usually omitted: Two moles magnesium react with one mole oxygen to produce two moles magnesium oxide.

The Mole Other Examples: Cu(s) + 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2(aq) One mole of copper metal reacts with two moles of silver nitrate solution to produce two moles of silver metal and one mole of copper(II) nitrate solution.

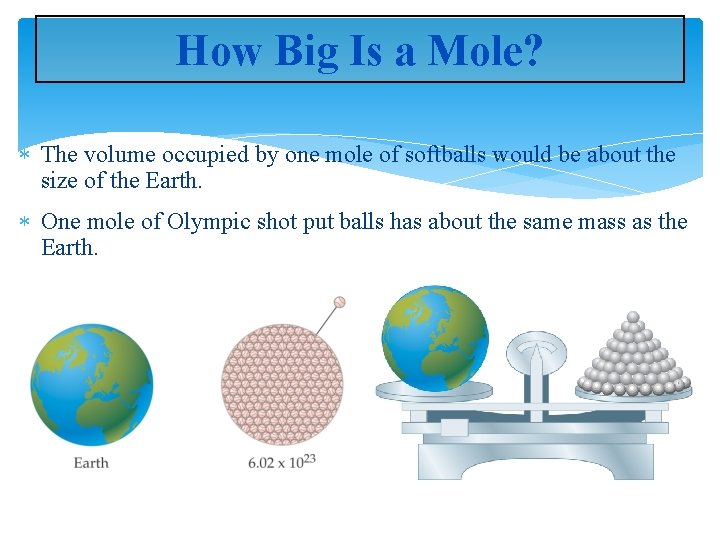
How Big Is a Mole? The volume occupied by one mole of softballs would be about the size of the Earth. One mole of Olympic shot put balls has about the same mass as the Earth.

Molar Mass What is the molar mass of KCl? What is the molar mass of Ba(NO 3)2?

Molar Mass What is the molar mass of Cu. SO 4 • 5 H 2 O?

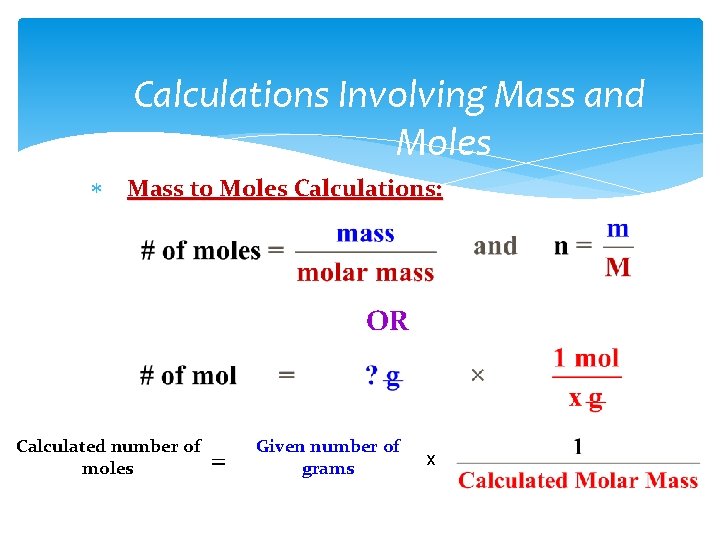
Calculations Involving Mass and Moles Mass to Moles Calculations: OR Calculated number of moles = Given number of grams x

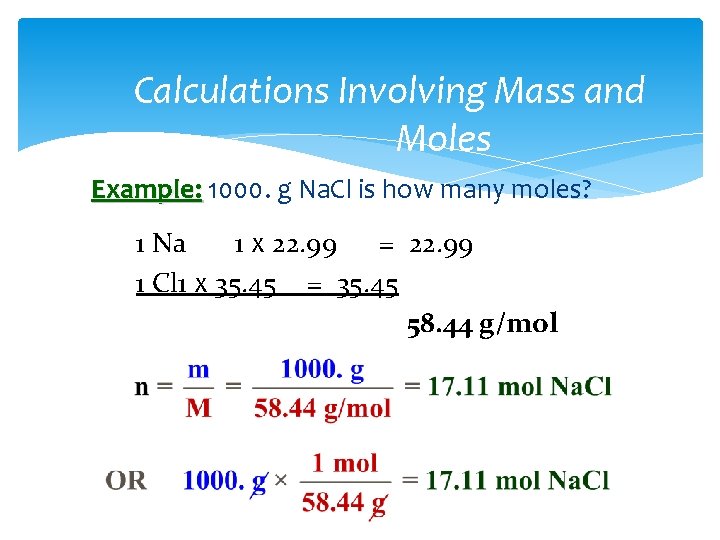
Calculations Involving Mass and Moles Example: 1000. g Na. Cl is how many moles? 1 Na 1 x 22. 99 = 22. 99 1 Cl 1 x 35. 45 = 35. 45 58. 44 g/mol

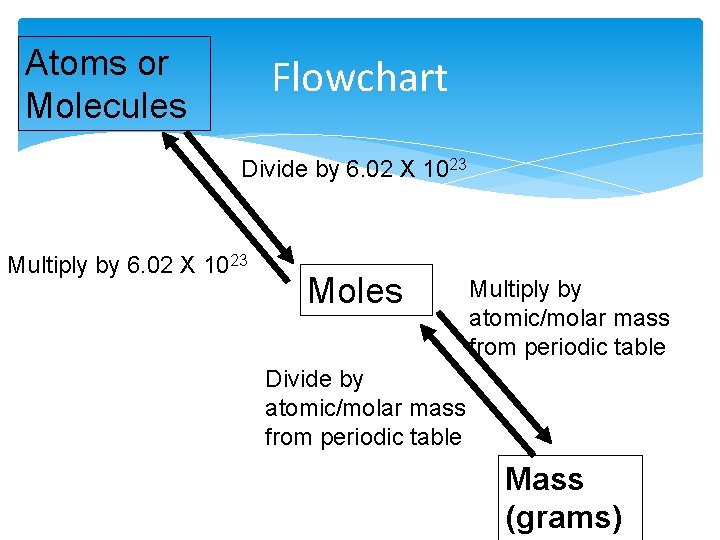
Atoms or Molecules Flowchart Divide by 6. 02 X 1023 Multiply by 6. 02 X 1023 Moles Multiply by atomic/molar mass from periodic table Divide by atomic/molar mass from periodic table Mass (grams)

Calculations molar mass Grams Avogadro’s number Moles particles Everything must go through Moles!!!

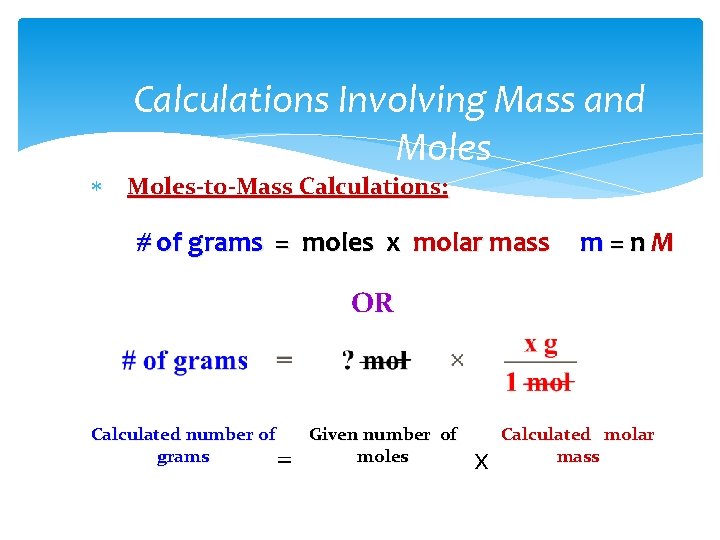
Calculations Involving Mass and Moles-to-Mass Calculations: # of grams = moles x molar mass m=n. M OR Calculated number of Given number of grams moles = x Calculated molar mass

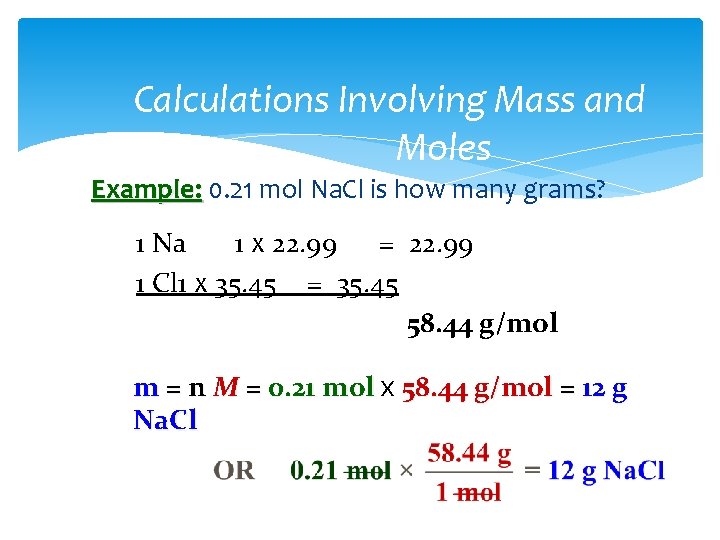
Calculations Involving Mass and Moles Example: 0. 21 mol Na. Cl is how many grams? 1 Na 1 x 22. 99 = 22. 99 1 Cl 1 x 35. 45 = 35. 45 58. 44 g/mol m = n M = 0. 21 mol x 58. 44 g/mol = 12 g Na. Cl

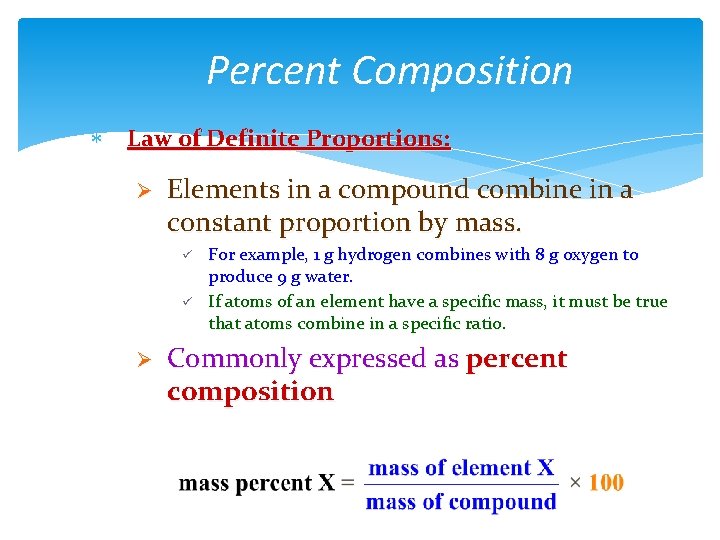
Percent Composition Law of Definite Proportions: Ø Elements in a compound combine in a constant proportion by mass. ü ü Ø For example, 1 g hydrogen combines with 8 g oxygen to produce 9 g water. If atoms of an element have a specific mass, it must be true that atoms combine in a specific ratio. Commonly expressed as percent composition

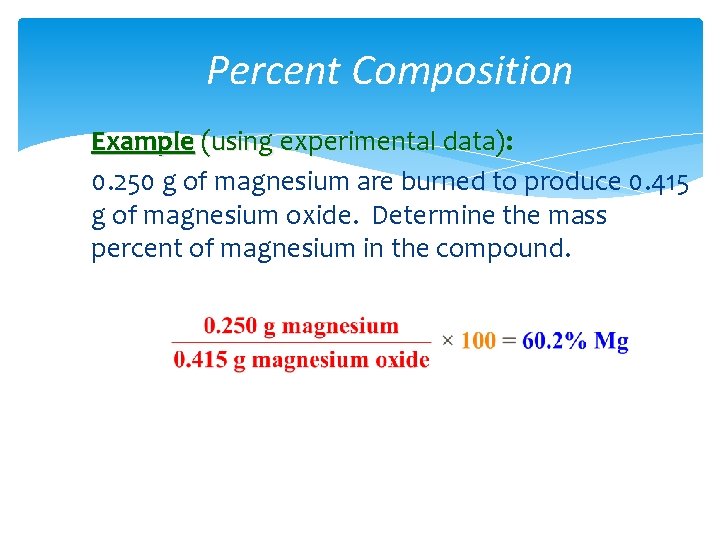
Percent Composition Example (using experimental data): 0. 250 g of magnesium are burned to produce 0. 415 g of magnesium oxide. Determine the mass percent of magnesium in the compound.

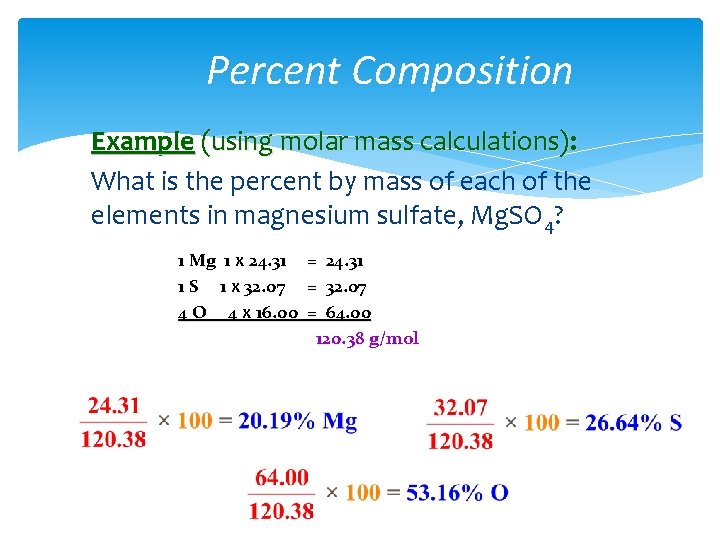
Percent Composition Example (using molar mass calculations): What is the percent by mass of each of the elements in magnesium sulfate, Mg. SO 4? 1 Mg 1 x 24. 31 = 24. 31 1 S 1 x 32. 07 = 32. 07 4 O 4 x 16. 00 = 64. 00 120. 38 g/mol

4. Combustion analysis of 0. 500 g of an unknown hydrocarbon yielded 1. 541 g of CO 2 and 0. 710 g H 2 O. a) What is the percent by mass of carbon in the compound?

Empirical/Molecular Formulas and REVIEW

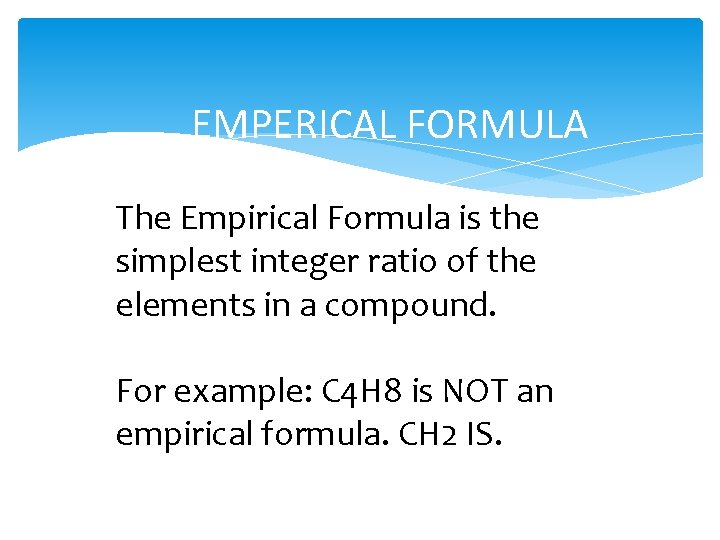
EMPERICAL FORMULA The Empirical Formula is the simplest integer ratio of the elements in a compound. For example: C 4 H 8 is NOT an empirical formula. CH 2 IS.

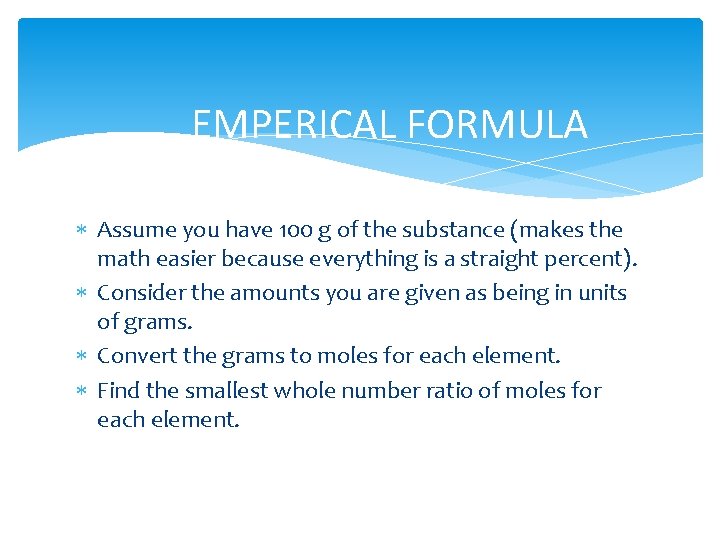
EMPERICAL FORMULA Assume you have 100 g of the substance (makes the math easier because everything is a straight percent). Consider the amounts you are given as being in units of grams. Convert the grams to moles for each element. Find the smallest whole number ratio of moles for each element.

EMPIRICAL FORMULA EXAMPLE: Find the empirical formula for a compound consisting of 63% Mn and 37% O

EMPIRICAL FORMULA Assuming 100 g of the compound, there would be 63 g Mn and 37 g O Look up the number of grams per mole for each element using the Periodic Table. There are 54. 94 grams in each mole of manganese and 16. 00 grams in a mole of oxygen. 63 g Mn × (1 mol Mn)/(54. 94 g Mn) = 1. 1 mol Mn 37 g O × (1 mol O)/(16. 00 g O) = 2. 3 mol O

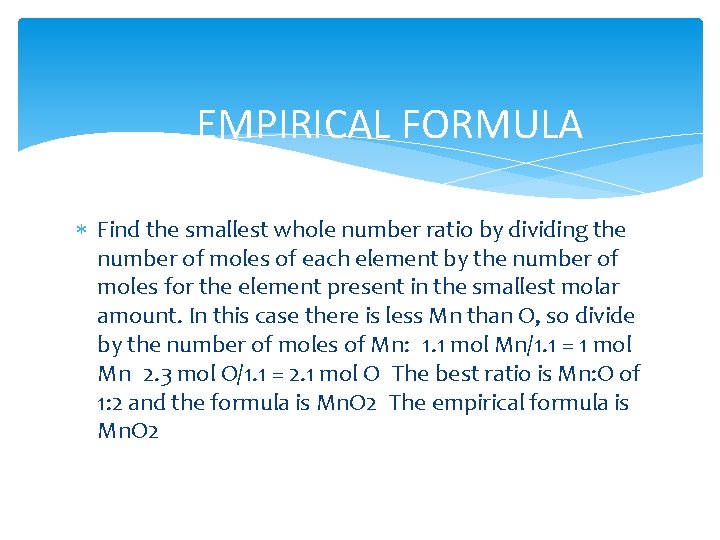
EMPIRICAL FORMULA Find the smallest whole number ratio by dividing the number of moles of each element by the number of moles for the element present in the smallest molar amount. In this case there is less Mn than O, so divide by the number of moles of Mn: 1. 1 mol Mn/1. 1 = 1 mol Mn 2. 3 mol O/1. 1 = 2. 1 mol O The best ratio is Mn: O of 1: 2 and the formula is Mn. O 2 The empirical formula is Mn. O 2

Molecular Formula is the exact ratio of the elements in the compound. You can find the Molecular Formula of a compound by using the empirical formula and the molar mass. The mass of the molecular formula is called the molecular mass (in the same way that the mass of the empirical formula is called the formula mass)

Molecular Formula 1) Calculate the mass of the empirical formula and divide the molar mass of the compound by the mass of the empirical formula in order to find the ratio between the molecular formula and the empirical formula. 2)Then multiply all the atoms by this ratio to find the molecular formula!

Molecular Formula EXAMPLE: What is the molecular formula of a compound that has a molecular weight of 240 g? A 5. 00 g sample contains 2. 00 g of Carbon, 0. 34 g of Hydrogen and 2. 69 g of Oxygen.