Unit 6 Stoichiometry Section 1 Mole and Gram







- Slides: 20

Unit 6: Stoichiometry Section 1: Mole and Gram: Gram

Stoichiometry • Technical word for the relationships among balanced equations, moles, and grams – Chemists use stoichiometry like cooks use cooking recipes

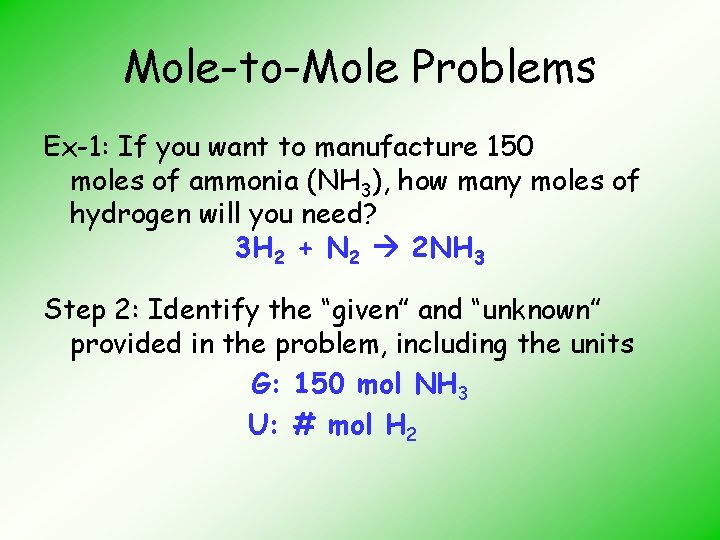
Mole-to-Mole Problems Ex-1: If you want to manufacture 150 moles of ammonia (NH 3), how many moles of hydrogen will you need? H 2 + N 2 NH 3 Step 1: Balance the equation 3 H 2 + N 2 2 NH 3

Mole-to-Mole Problems Ex-1: If you want to manufacture 150 moles of ammonia (NH 3), how many moles of hydrogen will you need? 3 H 2 + N 2 2 NH 3 Step 2: Identify the “given” and “unknown” provided in the problem, including the units G: 150 mol NH 3 U: # mol H 2

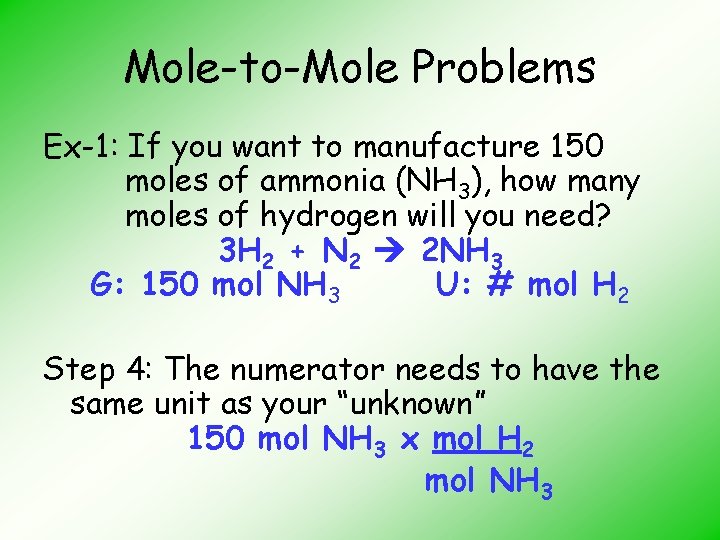
Mole-to-Mole Problems Ex-1: If you want to manufacture 150 moles of ammonia (NH 3), how many moles of hydrogen will you need? 3 H 2 + N 2 2 NH 3 G: 150 mol NH 3 U: # mol H 2 Step 3: Begin with the “given” # then choose a conversion factor that has in its denominator the same units as those in the “given” 150 mol NH 3 x _______ mol NH 3

Mole-to-Mole Problems Ex-1: If you want to manufacture 150 moles of ammonia (NH 3), how many moles of hydrogen will you need? 3 H 2 + N 2 2 NH 3 G: 150 mol NH 3 U: # mol H 2 Step 4: The numerator needs to have the same unit as your “unknown” 150 mol NH 3 x mol H 2 mol NH 3

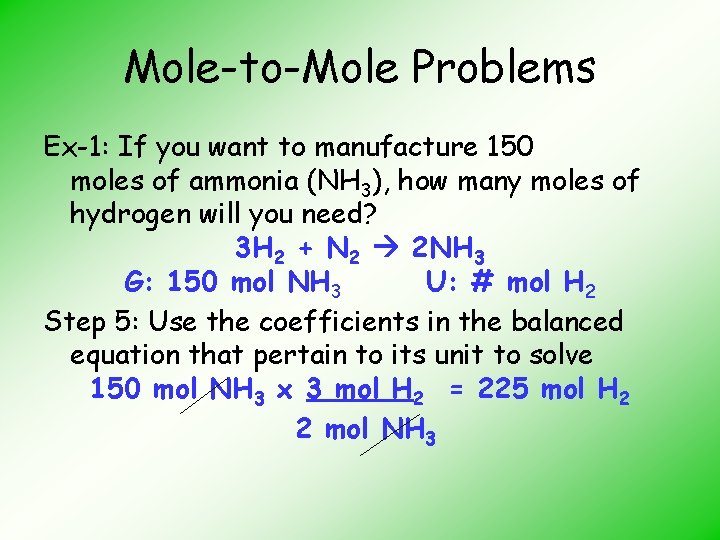
Mole-to-Mole Problems Ex-1: If you want to manufacture 150 moles of ammonia (NH 3), how many moles of hydrogen will you need? 3 H 2 + N 2 2 NH 3 G: 150 mol NH 3 U: # mol H 2 Step 5: Use the coefficients in the balanced equation that pertain to its unit to solve 150 mol NH 3 x 3 mol H 2 = 225 mol H 2 2 mol NH 3

Mole-to-Mole Problems Ex-1: If you want to manufacture 150 moles of ammonia (NH 3), how many moles of hydrogen will you need? 3 H 2 + N 2 2 NH 3 G: 150 mol NH 3 U: # mol H 2 Step 6: Make sure your final answer is in the correct # of sig figs with its unit 150 mol NH 3 x 3 mol H 2 = 225 mol H 2 230 mol H 2 2 mol NH 3

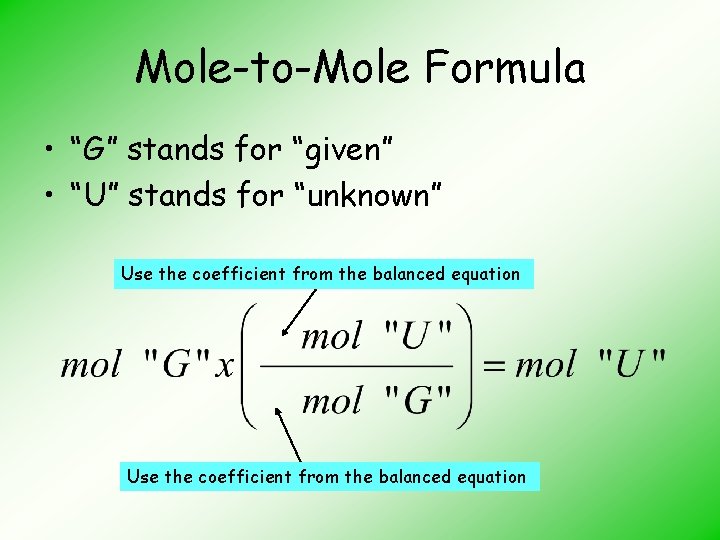
Mole-to-Mole Formula • “G” stands for “given” • “U” stands for “unknown” Use the coefficient from the balanced equation

Mole-to-Mole Practice Ex-2: If you want to make 100 moles of ammonia (NH 3), how many moles of nitrogen (N 2) will you need? H 2 + N 2 NH 3 50 mol N 2

Mole-to-Mole Practice Ex-3: If you have 36 moles of nitrogen (N 2), how many moles of hydrogen (H 2) will you need? H 2 + N 2 NH 3 110 mol H 2 or 1. 1 x 102 mol H 2

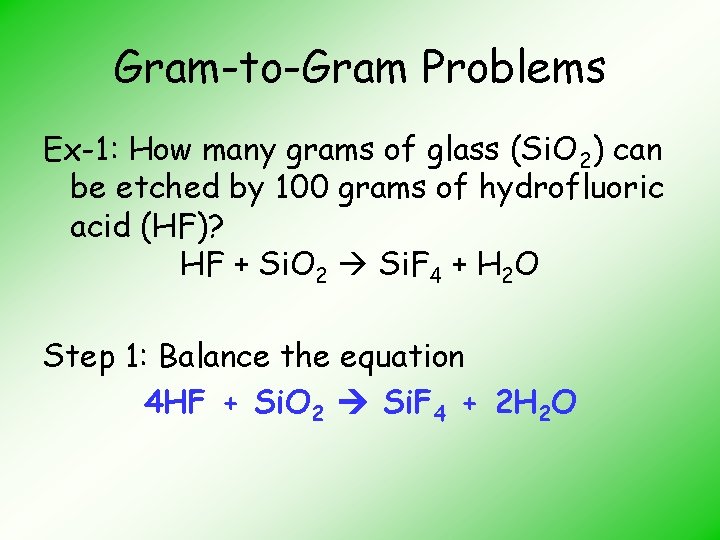
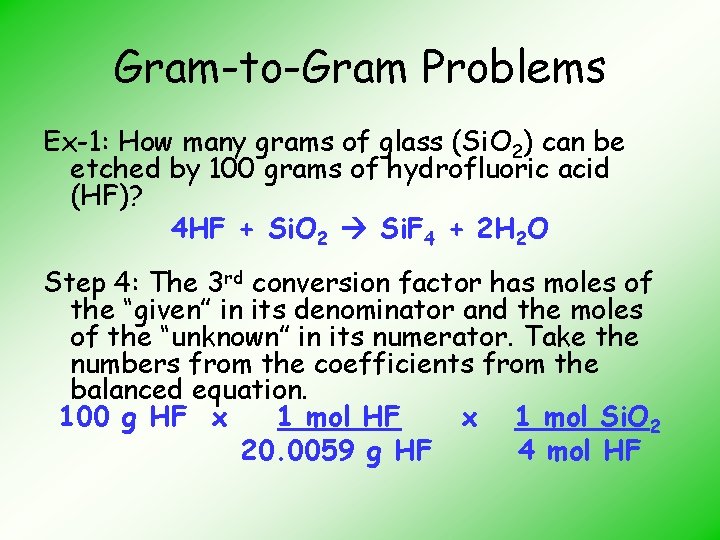
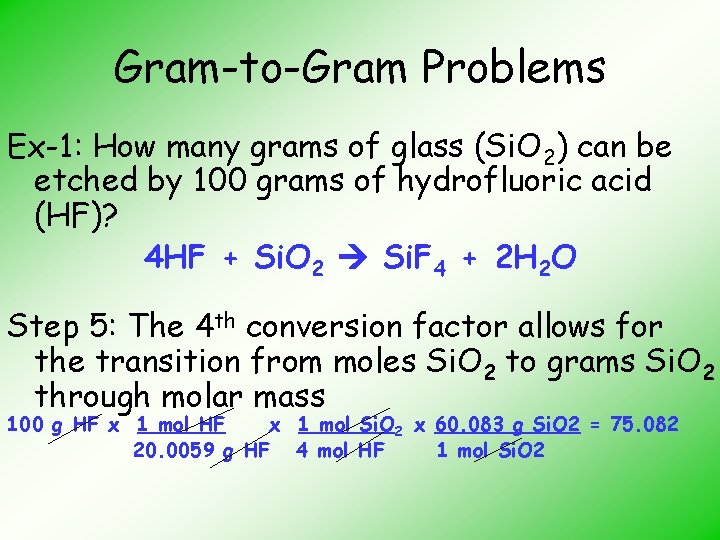
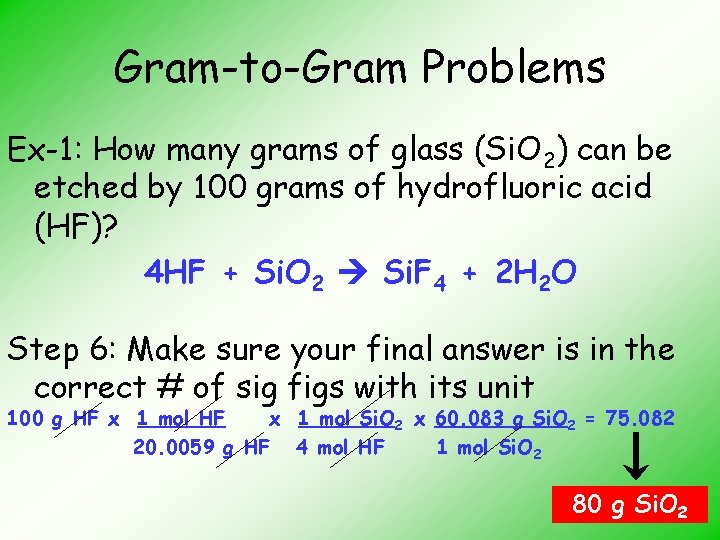
Gram-to-Gram Problems Ex-1: How many grams of glass (Si. O 2) can be etched by 100 grams of hydrofluoric acid (HF)? HF + Si. O 2 Si. F 4 + H 2 O Step 1: Balance the equation 4 HF + Si. O 2 Si. F 4 + 2 H 2 O

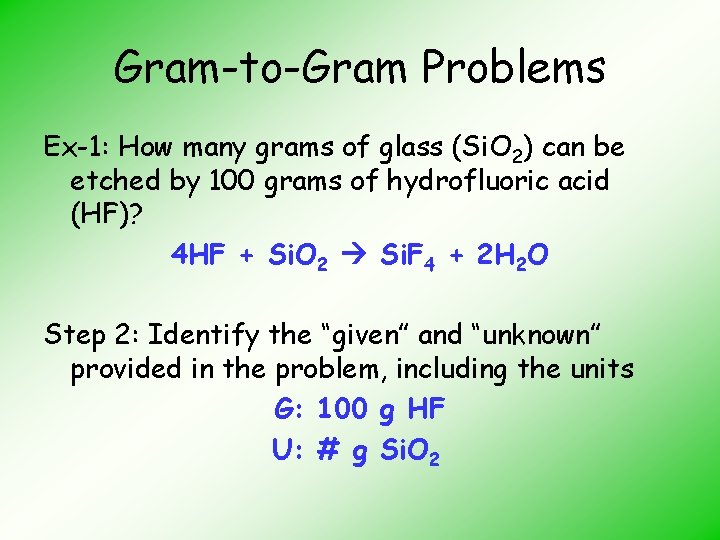
Gram-to-Gram Problems Ex-1: How many grams of glass (Si. O 2) can be etched by 100 grams of hydrofluoric acid (HF)? 4 HF + Si. O 2 Si. F 4 + 2 H 2 O Step 2: Identify the “given” and “unknown” provided in the problem, including the units G: 100 g HF U: # g Si. O 2

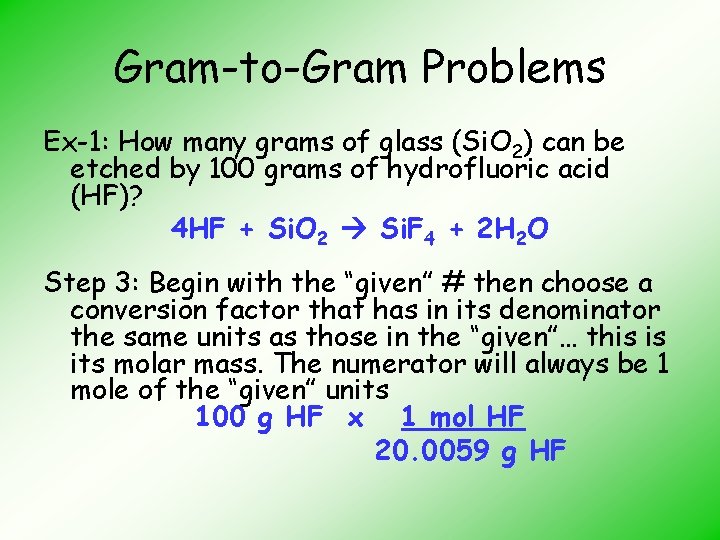
Gram-to-Gram Problems Ex-1: How many grams of glass (Si. O 2) can be etched by 100 grams of hydrofluoric acid (HF)? 4 HF + Si. O 2 Si. F 4 + 2 H 2 O Step 3: Begin with the “given” # then choose a conversion factor that has in its denominator the same units as those in the “given”… this is its molar mass. The numerator will always be 1 mole of the “given” units 100 g HF x 1 mol HF 20. 0059 g HF

Gram-to-Gram Problems Ex-1: How many grams of glass (Si. O 2) can be etched by 100 grams of hydrofluoric acid (HF)? 4 HF + Si. O 2 Si. F 4 + 2 H 2 O Step 4: The 3 rd conversion factor has moles of the “given” in its denominator and the moles of the “unknown” in its numerator. Take the numbers from the coefficients from the balanced equation. 100 g HF x 1 mol Si. O 2 20. 0059 g HF 4 mol HF

Gram-to-Gram Problems Ex-1: How many grams of glass (Si. O 2) can be etched by 100 grams of hydrofluoric acid (HF)? 4 HF + Si. O 2 Si. F 4 + 2 H 2 O Step 5: The 4 th conversion factor allows for the transition from moles Si. O 2 to grams Si. O 2 through molar mass 100 g HF x 1 mol Si. O 2 x 60. 083 g Si. O 2 = 75. 082 20. 0059 g HF 4 mol HF 1 mol Si. O 2

Gram-to-Gram Problems Ex-1: How many grams of glass (Si. O 2) can be etched by 100 grams of hydrofluoric acid (HF)? 4 HF + Si. O 2 Si. F 4 + 2 H 2 O Step 6: Make sure your final answer is in the correct # of sig figs with its unit 100 g HF x 1 mol Si. O 2 x 60. 083 g Si. O 2 = 75. 082 20. 0059 g HF 4 mol HF 1 mol Si. O 2 80 g Si. O 2

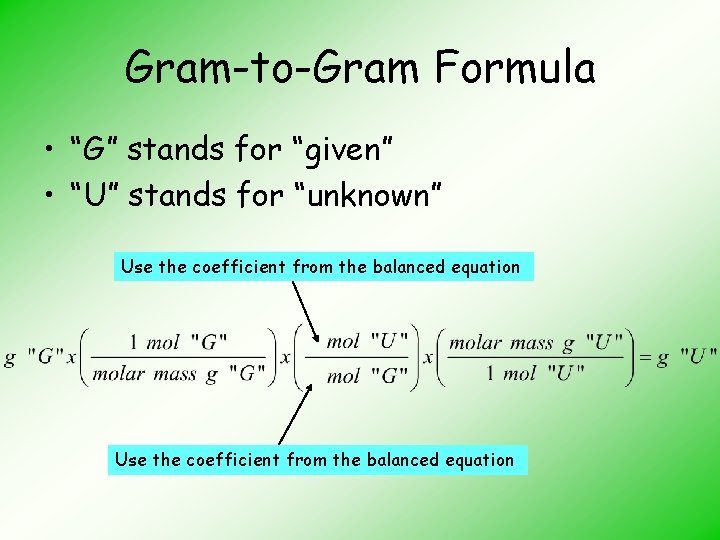
Gram-to-Gram Formula • “G” stands for “given” • “U” stands for “unknown” Use the coefficient from the balanced equation

Gram-to-Gram Practice Ex-2: How many grams of glass (Si. O 2) can be made from 230 grams of H 2 O? 4 HF + Si. O 2 Si. F 4 + 2 H 2 O 380 g H 2 O

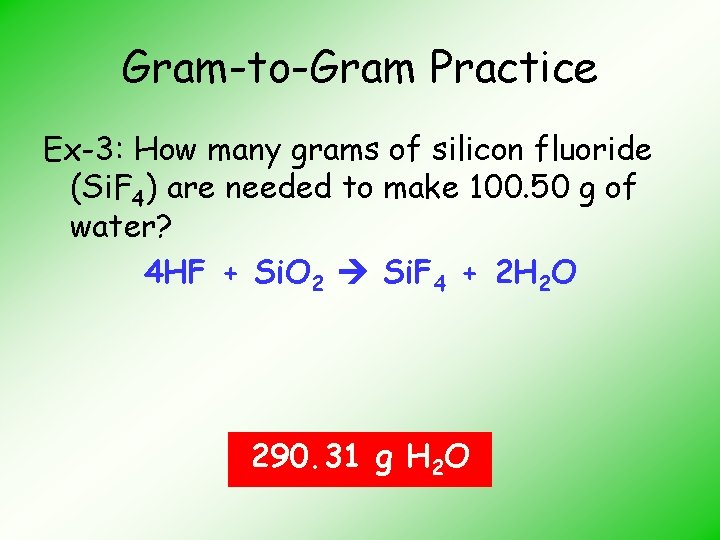
Gram-to-Gram Practice Ex-3: How many grams of silicon fluoride (Si. F 4) are needed to make 100. 50 g of water? 4 HF + Si. O 2 Si. F 4 + 2 H 2 O 290. 31 g H 2 O