Unit 6 Lesson 8 Ionic Bonding Ionic Bonds

Unit 6, Lesson 8: Ionic Bonding
Ionic Bonds • An ionic bond is formed by the electrostatic attraction between positive and negative ions. • Ionic bonds typically form between metals and non-metals. • The greater the radius of the ions, the _____ the ionic bond strength. • The greater the ion charges, the _____ the ionic bond strength.

Pick-a-Side: Ionic Bond or No? • • • Ba and S P and Cl Ca and O Rb and I O and H S and O

Electronegativity • The electronegativity of an atom is its tendency to attract electrons from a neighbouring atom. • Going down the periodic table, atoms have a _____ radius, and thus a _____ attraction to the outer electrons of another atom. • Going left to right, atoms have a _____ radius, and thus a _____ attraction to the outer electrons of another atom.
Electronegativity and Ionization Energy • An atom with a high electronegativity _____ attracts electrons, and has a _____ ionization energy. • An atom with a low electronegativity _____ attracts electrons, and has a _____ ionization energy.
Brain Break!
Predicting Ion Charges • In general, when an atom forms an ion, it loses or gains sufficient electrons to obtain a closed shell.
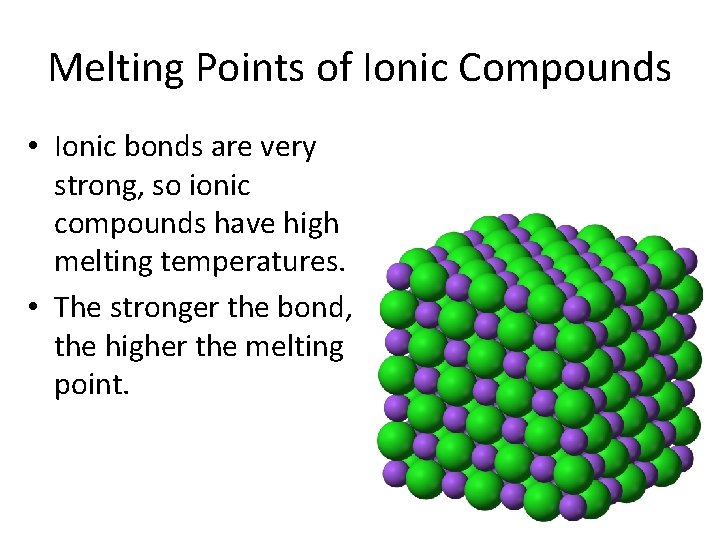
Melting Points of Ionic Compounds • Ionic bonds are very strong, so ionic compounds have high melting temperatures. • The stronger the bond, the higher the melting point.

Pick-a-Side: Which will have a higher melting point? • • • Ca. O or Rb. I Be. O or BN Li. F or Na. Cl Cs. Cl or Ba. S Rb. I or KCl Be. O or Mg. S
The Size of Ions • When electrons are added to an atom, the amount of electrostatic repulsion among electrons _____, so the atom gets _____. • When electrons are removed from an atom, the amount of electrostatic repulsion among electrons _____, so the atom gets _____.

Practice: • Pg. 182 #79, 80, and 83
- Slides: 12