Unit 6 Lesson 2 Synthesis Decomposition Combustion Types
Unit 6 – Lesson 2 Synthesis, Decomposition &Combustion
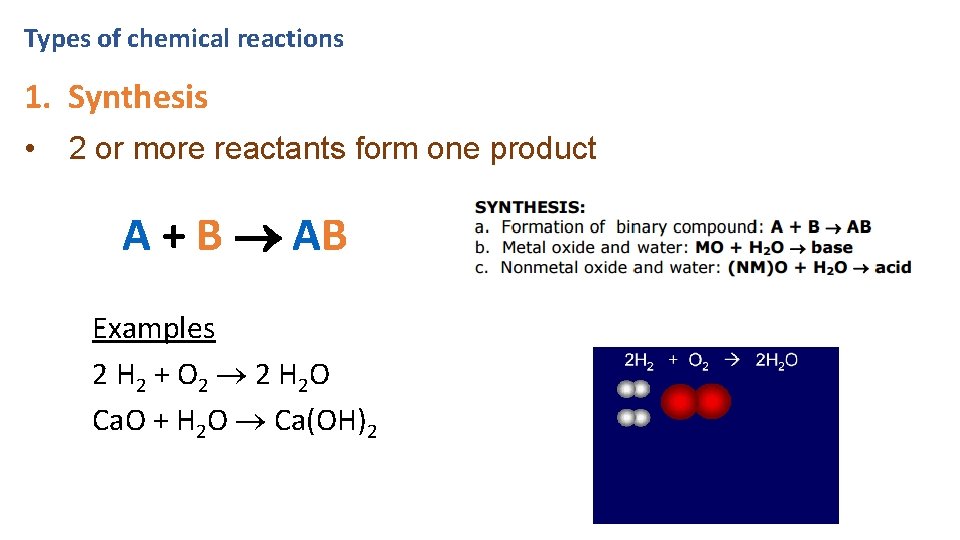
Types of chemical reactions 1. Synthesis • 2 or more reactants form one product A + B AB Examples 2 H 2 + O 2 2 H 2 O Ca. O + H 2 O Ca(OH)2

Nonmetal Oxide and Water • SO 3 (g) + H 2 O (l) → H 2 SO 4 (aq) • Sulfur trioxide reacts with water to form sulfuric acid.

Predict the products of a synthesis reaction between Magnesium and Oxygen is a diatomic! Mg 2+ O 2 - Mg + O 2 → Mg. O Balance the equation: 2 Mg + O 2 → 2 Mg. O You should NOT balance the equation and predict products at the same time, they are separate processes
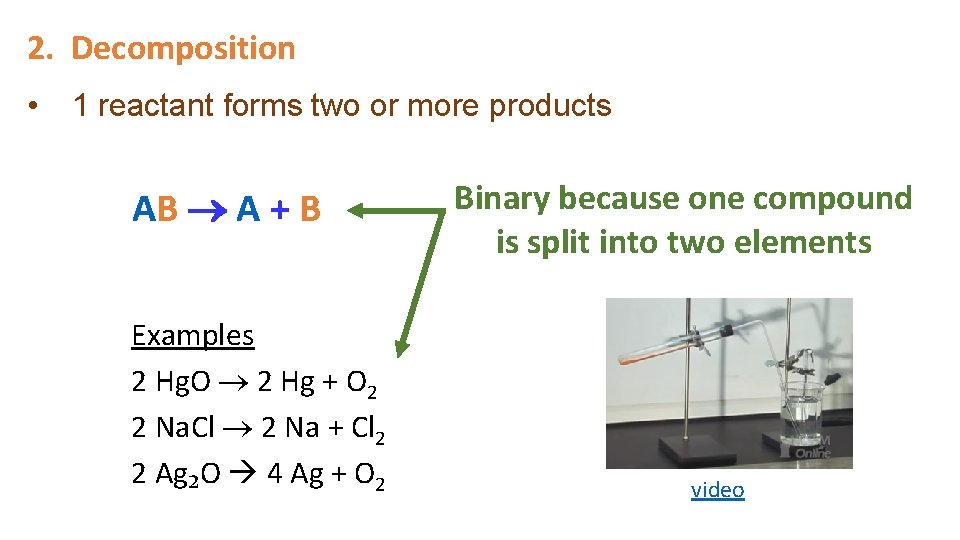
2. Decomposition • 1 reactant forms two or more products AB A + B Examples 2 Hg. O 2 Hg + O 2 2 Na. Cl 2 Na + Cl 2 2 Ag₂O 4 Ag + O 2 Binary because one compound is split into two elements video
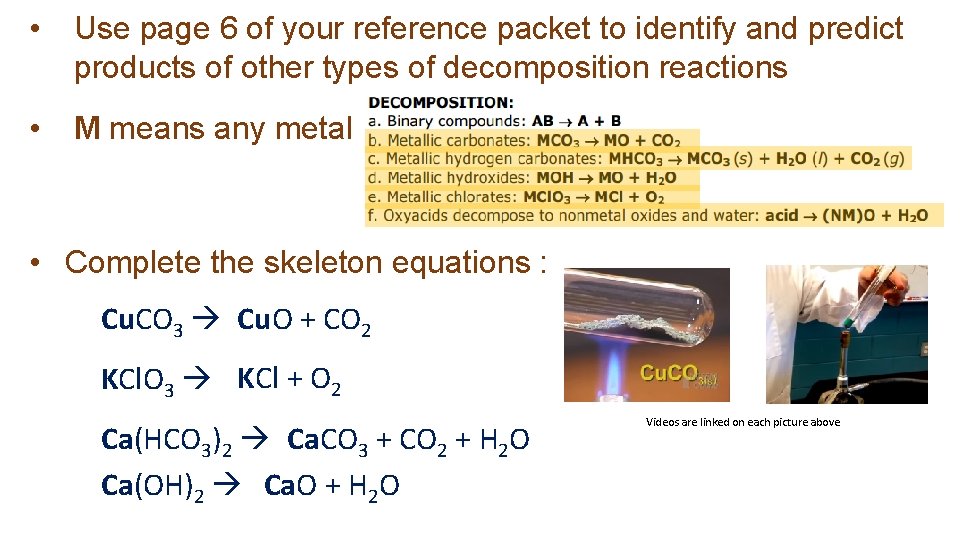
• Use page 6 of your reference packet to identify and predict products of other types of decomposition reactions • M means any metal • Complete the skeleton equations : Cu. CO 3 Cu. O + CO 2 KCl. O 3 KCl + O 2 Ca(HCO 3)2 Ca. CO 3 + CO 2 + H 2 O Ca(OH)2 Ca. O + H 2 O Videos are linked on each picture above

Video of Decomposition Reaction Let’s practice balancing equations before we go on…

Copper(II) Oxide will decompose when heated strongly. 1. Give the word and formula equation. 2. Balance the equation Copper(II) oxide Copper + Oxygen Cu. O Cu + O 2 2 Cu. O 2 Cu + O 2
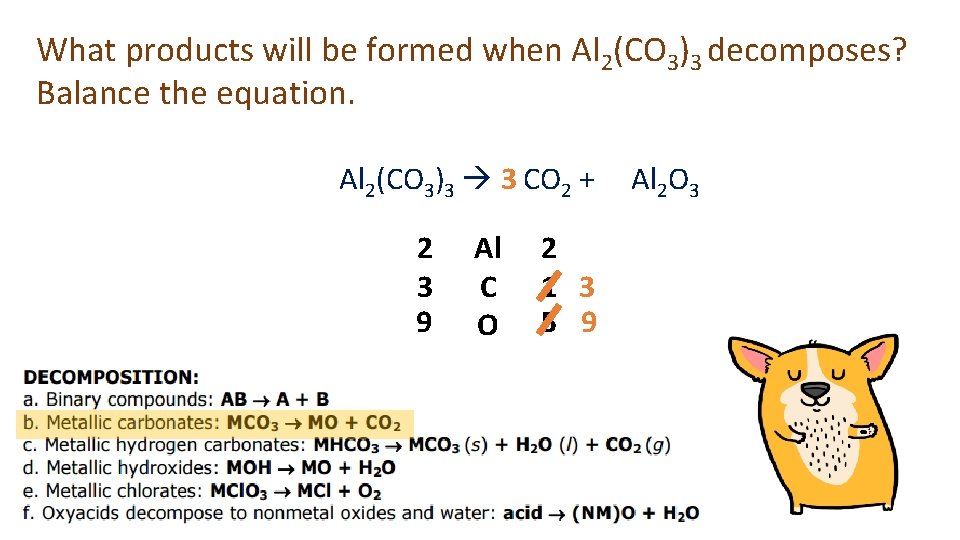
What products will be formed when Al 2(CO 3)3 decomposes? Balance the equation. Al 2(CO 3)3 3 CO 2 + 2 3 9 Al C O 2 1 3 5 9 Al 2 O 3

3. Combustion • When a compound (usually a hydrocarbon) reacts with oxygen producing water, carbon dioxide and heat. hydrocarbon compound made of H & C Examples No hydrocarbon: C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O Hydrocarbon: CH 4 + 2 O 2 CO 2 + 2 H 2 O
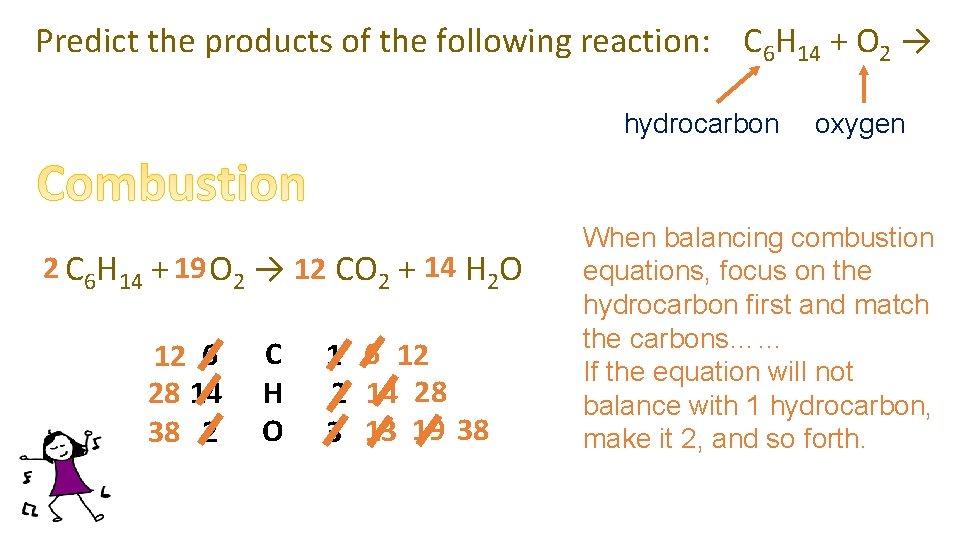
Predict the products of the following reaction: C 6 H 14 + O 2 → hydrocarbon 2 C 6 H 14 + 19 O 2 → 12 6 CO 2 + 14 7 H 2 O 12 6 28 14 38 2 C H O 1 6 12 2 14 28 3 13 19 38 oxygen When balancing combustion equations, focus on the hydrocarbon first and match the carbons…… If the equation will not balance with 1 hydrocarbon, make it 2, and so forth.
- Slides: 12