Unit 6 Lesson 2 Synthesis Decomposition Combustion Types
Unit 6 – Lesson 2 Synthesis, Decomposition &Combustion
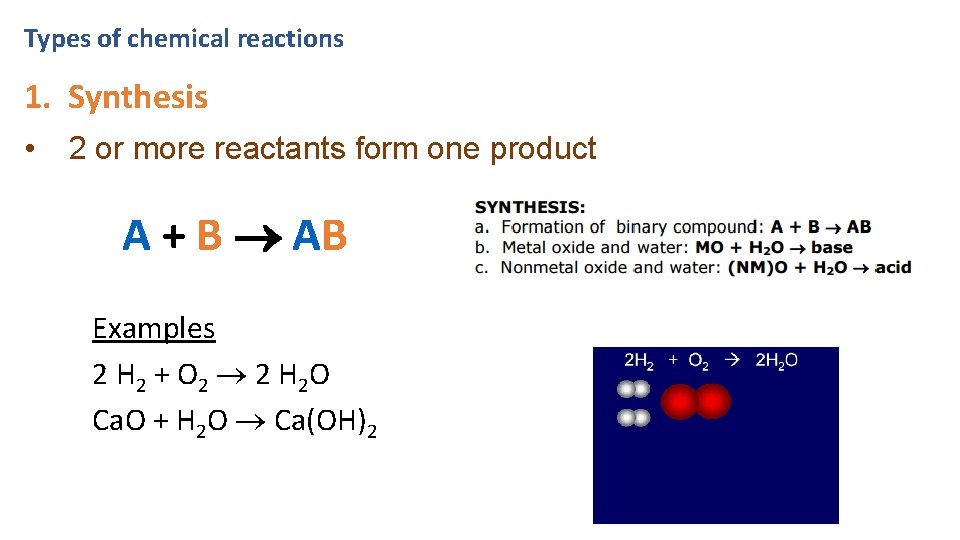
Types of chemical reactions 1. Synthesis • 2 or more reactants form one product A + B AB Examples 2 H 2 + O 2 2 H 2 O Ca. O + H 2 O Ca(OH)2
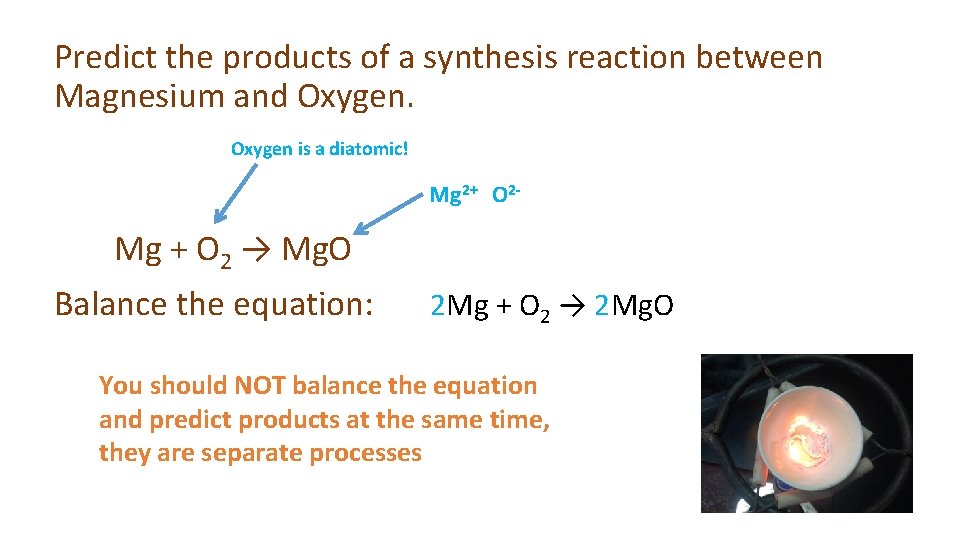
Predict the products of a synthesis reaction between Magnesium and Oxygen is a diatomic! Mg 2+ O 2 - Mg + O 2 → Mg. O Balance the equation: 2 Mg + O 2 → 2 Mg. O You should NOT balance the equation and predict products at the same time, they are separate processes
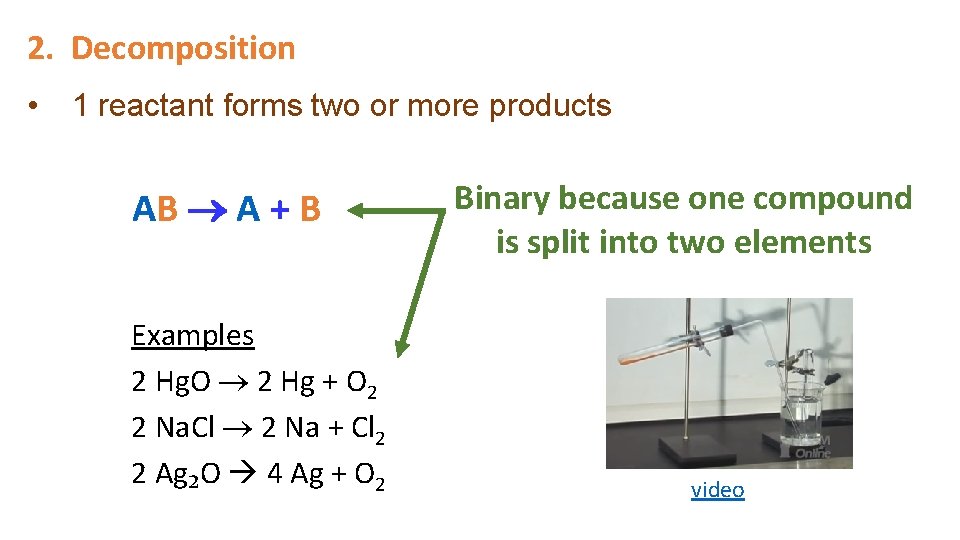
2. Decomposition • 1 reactant forms two or more products AB A + B Examples 2 Hg. O 2 Hg + O 2 2 Na. Cl 2 Na + Cl 2 2 Ag₂O 4 Ag + O 2 Binary because one compound is split into two elements video
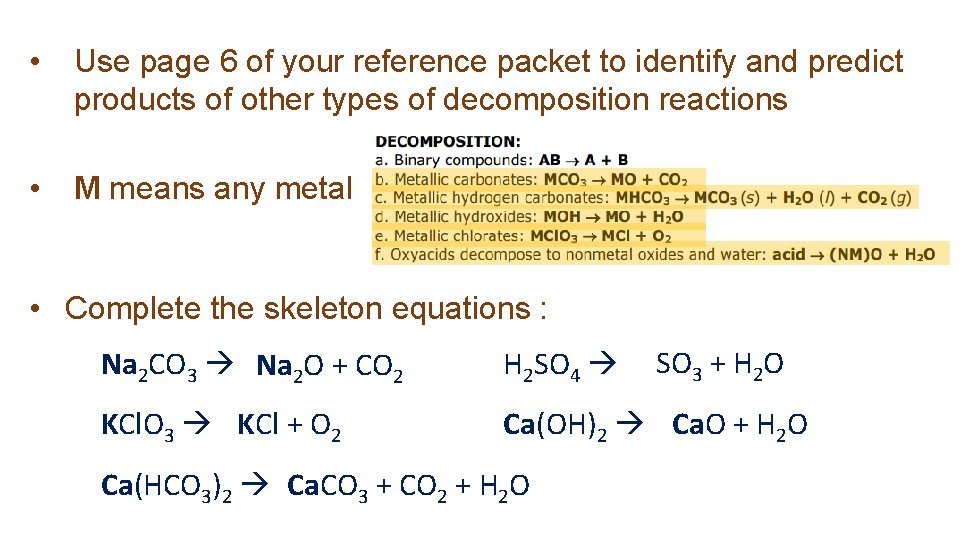
• Use page 6 of your reference packet to identify and predict products of other types of decomposition reactions • M means any metal • Complete the skeleton equations : SO 3 + H 2 O Na 2 CO 3 Na 2 O + CO 2 H 2 SO 4 KCl. O 3 KCl + O 2 Ca(OH)2 Ca. O + H 2 O Ca(HCO 3)2 Ca. CO 3 + CO 2 + H 2 O

Copper(II) Oxide will decompose when heated strongly. 1. Give the word and formula equation. 2. Balance the equation Copper(II) oxide Copper + Oxygen Cu. O Cu + O 2 2 Cu. O 2 Cu + O 2
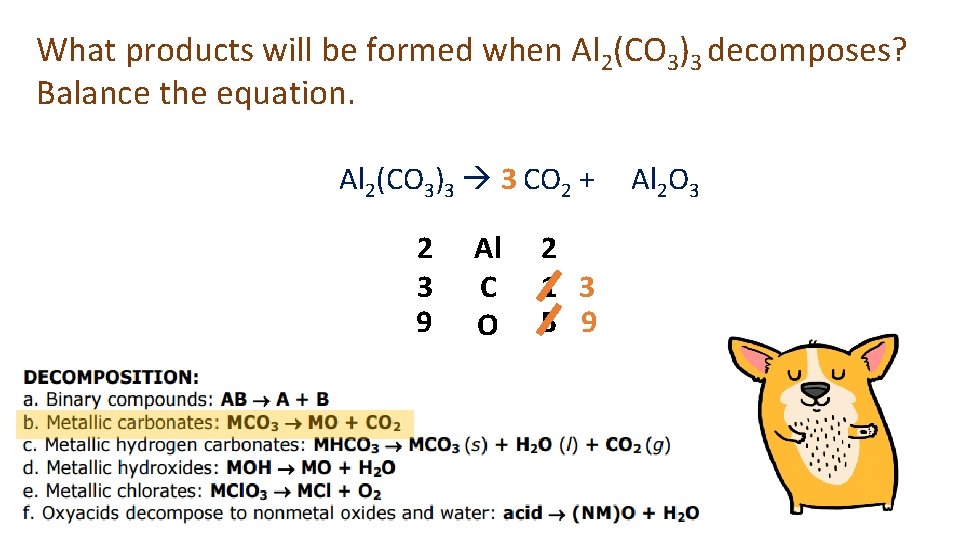
What products will be formed when Al 2(CO 3)3 decomposes? Balance the equation. Al 2(CO 3)3 3 CO 2 + 2 3 9 Al C O 2 1 3 5 9 Al 2 O 3

3. Combustion • When a compound (usually a hydrocarbon) reacts with oxygen producing water, carbon dioxide and heat. hydrocarbon compound made of H & C Examples No hydrocarbon: C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O Hydrocarbon: CH 4 + 2 O 2 CO 2 + 2 H 2 O
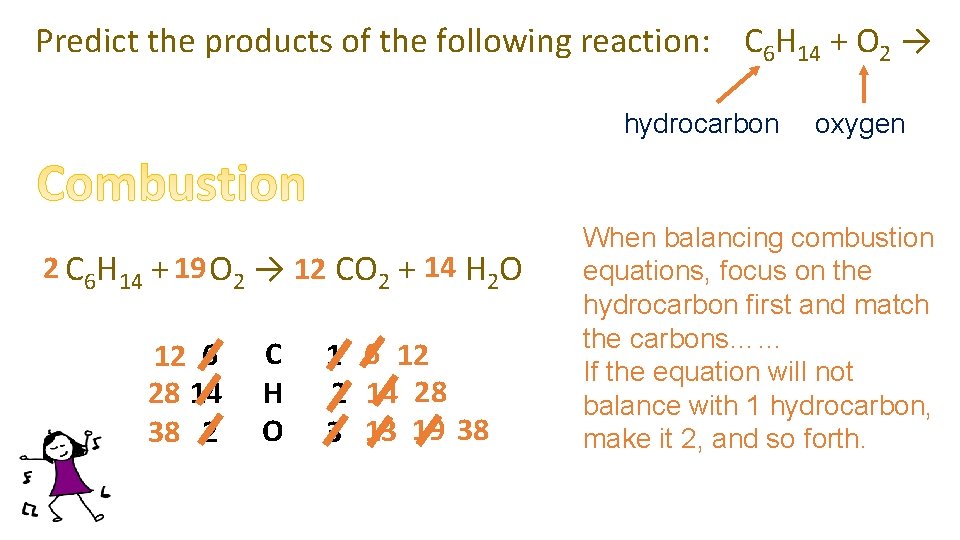
Predict the products of the following reaction: C 6 H 14 + O 2 → hydrocarbon 2 C 6 H 14 + 19 O 2 → 12 6 CO 2 + 14 7 H 2 O 12 6 28 14 38 2 C H O 1 6 12 2 14 28 3 13 19 38 oxygen When balancing combustion equations, focus on the hydrocarbon first and match the carbons…… If the equation will not balance with 1 hydrocarbon, make it 2, and so forth.
- Slides: 9