Unit 6 Chemical Reactions Day 4 Notes Chemical





- Slides: 14

Unit 6: Chemical Reactions Day 4– Notes: Chemical Rxns and M&M Balancing

Warm Up OPEN: To Warm Up Section WRITE: Today’s date ANSWER THE FOLLOWING: 1. What was the ratio for the loudest explosion on Wednesday? 2. Why was it the loudest? 3. Why were the other two “quieter” explosions? TIME: 4 MINUTES WHEN DONE: Be ready to share out

Agenda • Toolbox Entry • Balancing Equations with Models: M&Ms

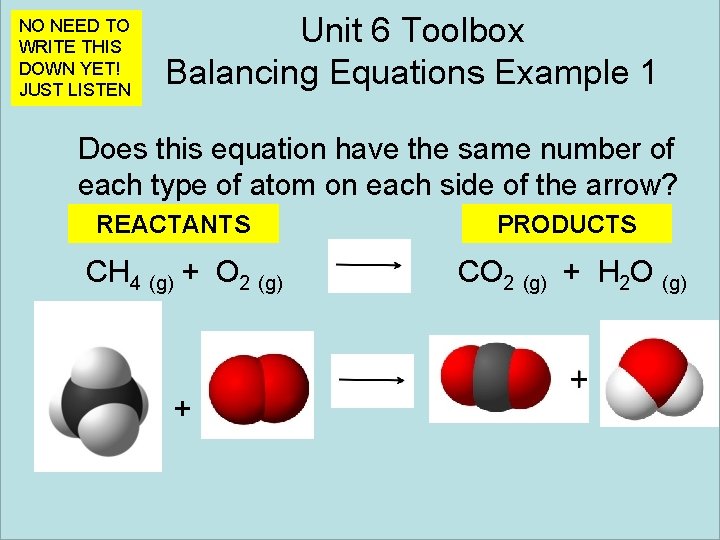
NO NEED TO WRITE THIS DOWN YET! JUST LISTEN Unit 6 Toolbox Balancing Equations Example 1 Does this equation have the same number of each type of atom on each side of the arrow? REACTANTS CH 4 (g) + O 2 (g) + PRODUCTS CO 2 (g) + H 2 O (g)

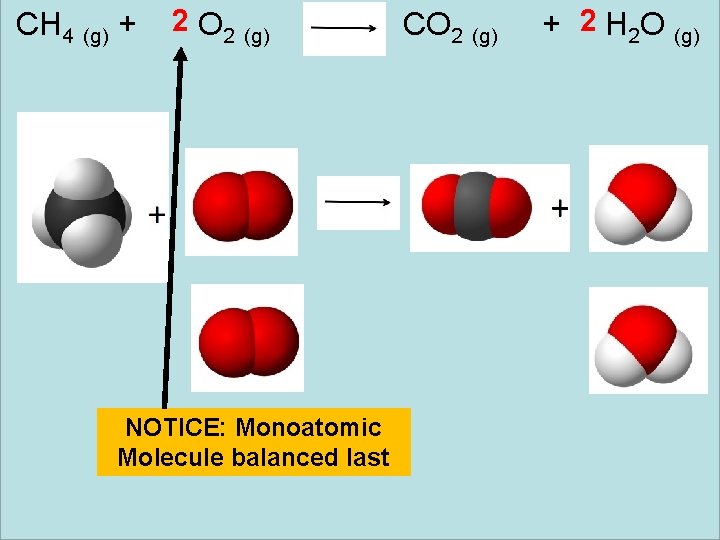
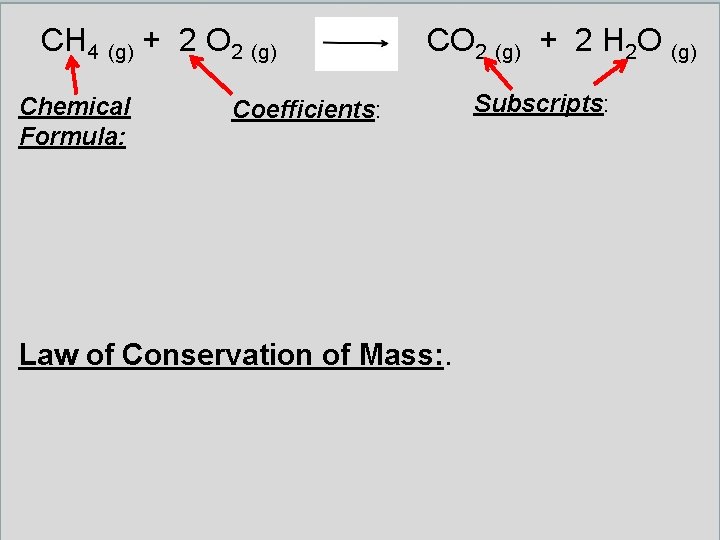
CH 4 (g) + 2 O 2 (g) NOTICE: Monoatomic Molecule balanced last CO 2 (g) + 2 H 2 O (g)

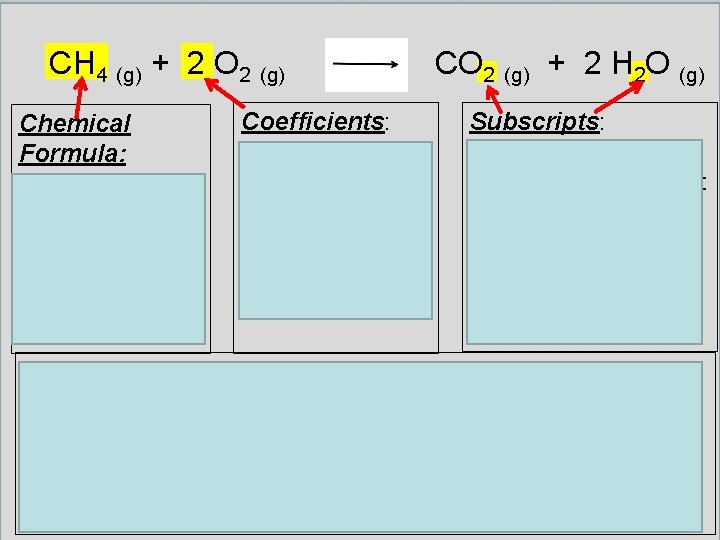
CH 4 (g) + 2 O 2 (g) Chemical Formula: Shows how many atoms of each element are in a substance Coefficients: numbers in front of a formula to show many of those compounds are needed (NUMBER CAN BE CHANGED) CO 2 (g) + 2 H 2 O (g) Subscripts: numbers below the element symbols that show many of those atoms are in the formula (NUMBER CANNOT BE CHANGED) Law of Conservation of Mass: Atoms are never created nor destroyed in a chemical reaction – only rearranged – and the number of atoms are the same on each side of the reaction. Therefore the mass before and the mass after the reaction are the same.

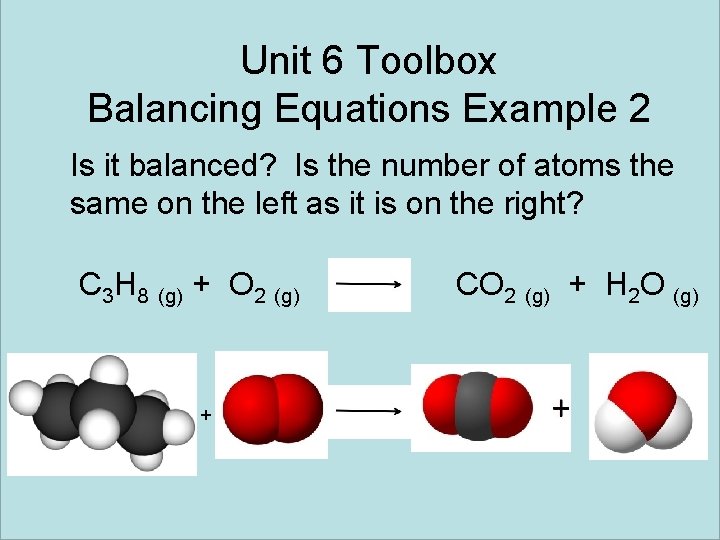
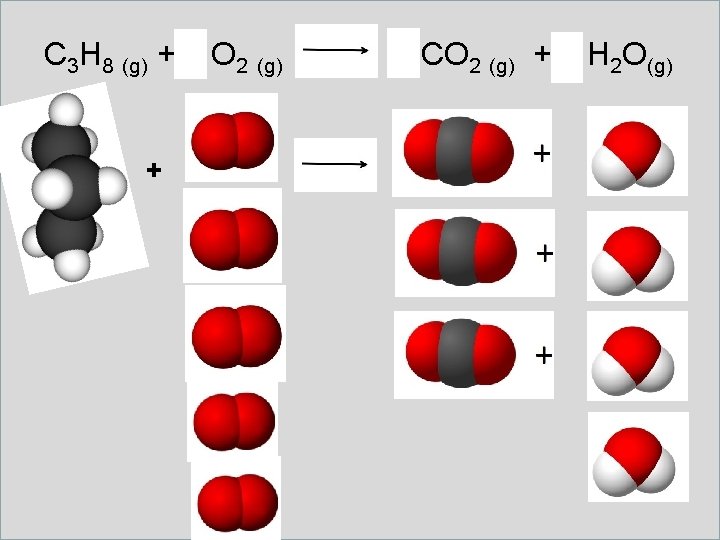
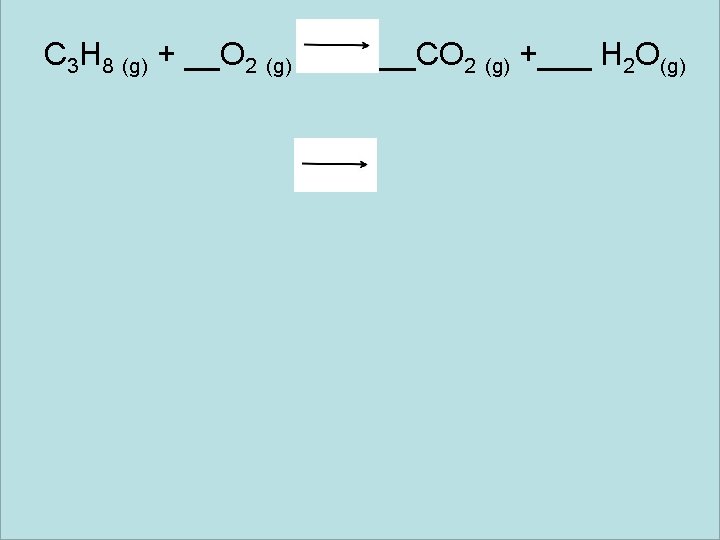
Unit 6 Toolbox Balancing Equations Example 2 Is it balanced? Is the number of atoms the same on the left as it is on the right? C 3 H 8 (g) + O 2 (g) + CO 2 (g) + H 2 O (g)

C 3 H 8 (g) + 5 O 2 (g) + 3 CO 2 (g) + 4 H 2 O(g)

Toolbox Entry FOLD: The two small pages in half GLUE: Down in Unit 6 Toolbox, so you have a “flap” (see example) TIME: 2 MINUTES WHEN DONE: Wash your hands

Lab: M&M Balancing WASH: Yo’ hands THEN: Send the tallest person up to the front to get yo’ bag o’ candy PUT: Pile of M&M’s on plate DO NOT EAT!!! TIME: 6 minutes WHEN DONE: Ask your table partner(s) about their favorite color

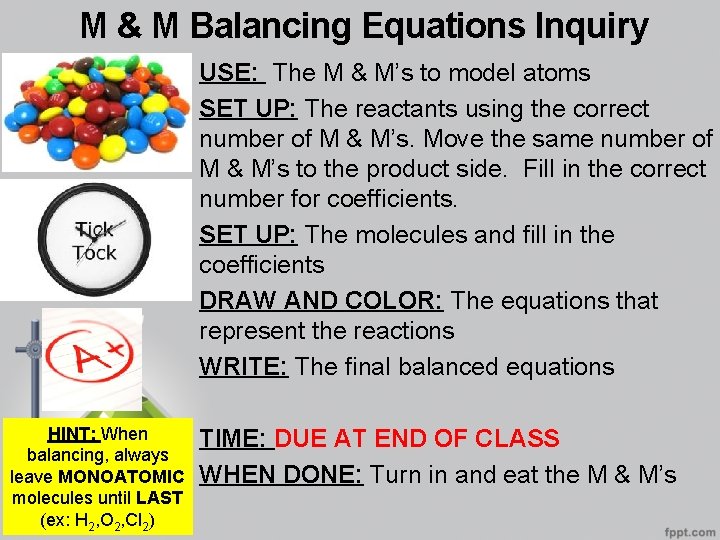
M & M Balancing Equations Inquiry USE: The M & M’s to model atoms SET UP: The reactants using the correct number of M & M’s. Move the same number of M & M’s to the product side. Fill in the correct number for coefficients. SET UP: The molecules and fill in the coefficients DRAW AND COLOR: The equations that represent the reactions WRITE: The final balanced equations HINT: When balancing, always leave MONOATOMIC molecules until LAST (ex: H 2, O 2, Cl 2) TIME: DUE AT END OF CLASS WHEN DONE: Turn in and eat the M & M’s

CH 4 (g) + 2 O 2 (g) Chemical Formula: CO 2 (g) + 2 H 2 O (g) Coefficients: Law of Conservation of Mass: . Subscripts:

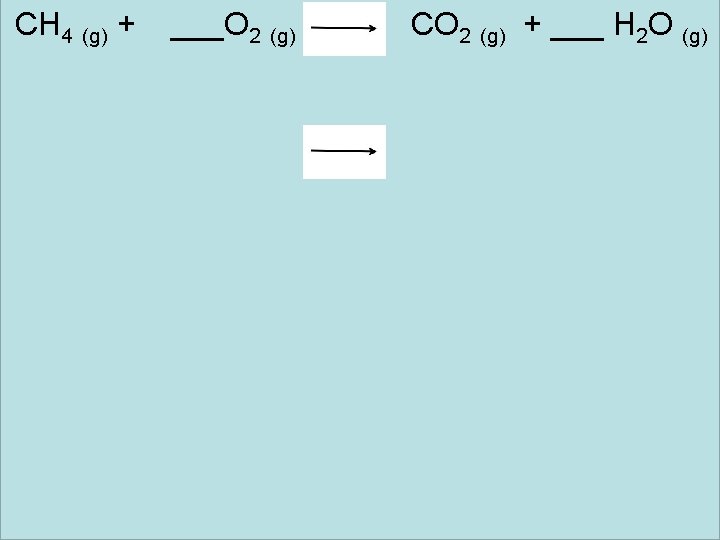
CH 4 (g) + ___O 2 (g) CO 2 (g) + ___ H 2 O (g)

C 3 H 8 (g) + __O 2 (g) __CO 2 (g) +___ H 2 O(g)