Unit 6 Chemical Reactions Day 1Notebook Check Water

Unit 6: Chemical Reactions Day 1–Notebook Check, Water Rocket, and Types of Reaction Web. Quest

Warm Up SET UP: Unit 7 in your notebook GLUE: In Learning Target Scale (in blue bin) MAKE: Unit 7 Warm up section • Next LEFT and RIGHT-HAND pages • Place Sticky Note in Warm Up Section MAKE: Unit 7 Toolbox • Next 2 LEFT and RIGHT-HAND pages TIME: 3 minutes WHEN DONE: Think about how we will make a water rocket

Agenda • Notebook Check • The Reaction between H 2 and O 2 – Observations – Inferences – Summary Conclusion • Thu/Mon: Speakers from Metro about Climate Change • Next Week: Begin Types of Chemical Reactions Web. Quest

Get Organized, Prioritize! DO: Unit 6 Notebook Check CHECK: Your own notebook for all items on handout IF YOU NEED TO: Write Summary or Add Questions TIME: 8 MINUTES WHEN DONE: Trade notebooks with a partner and have them verify that you have all items and turn in NOTEBOOK at end of class WITH NOTEBOOK CHECK SHEET tucked into it

Set Up Cornell Note OPEN TO: Next available right-hand page SET UP: Cornell note • Title: Hydrogen and Oxygen Reaction • Essential question: Which ratio of hydrogen and oxygen gas result in the biggest explosion and why? TIME: 2 minutes WHEN DONE: Make a prediction about ratios of H 2 to O 2

5/1 Reaction: Hydrogen and Oxygen EQ: Which ratio of hydrogen and oxygen gas result in the biggest explosion and why? In this demonstration, we will: • Collect H 2 gas in 2 -liter bottle in reaction between zinc and 3 M hydrochloric acid • Add O 2 from Oxygen tank • Test out best ratio of H 2 to O 2 to make the biggest explosion
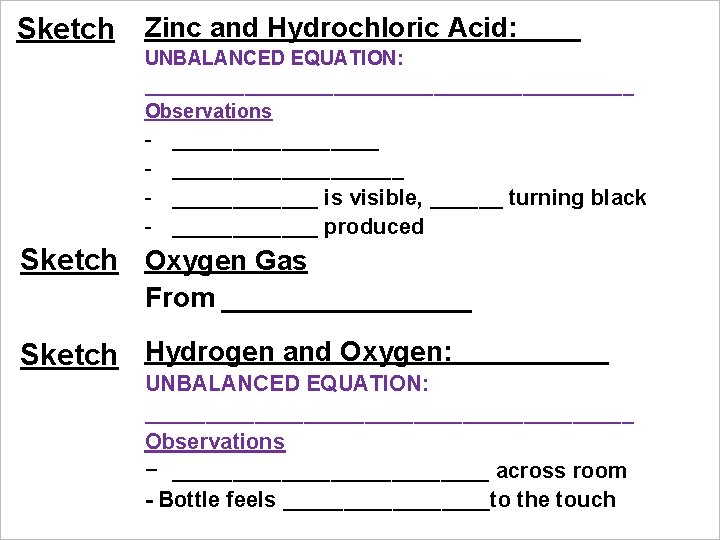
Sketch Zinc and Hydrochloric Acid: UNBALANCED EQUATION: ______________________ Observations - ___________________ is visible, ______ turning black ______ produced Sketch Oxygen Gas From ________ Sketch Hydrogen and Oxygen: UNBALANCED EQUATION: ____________________ Observations − _____________ across room - Bottle feels _________to the touch
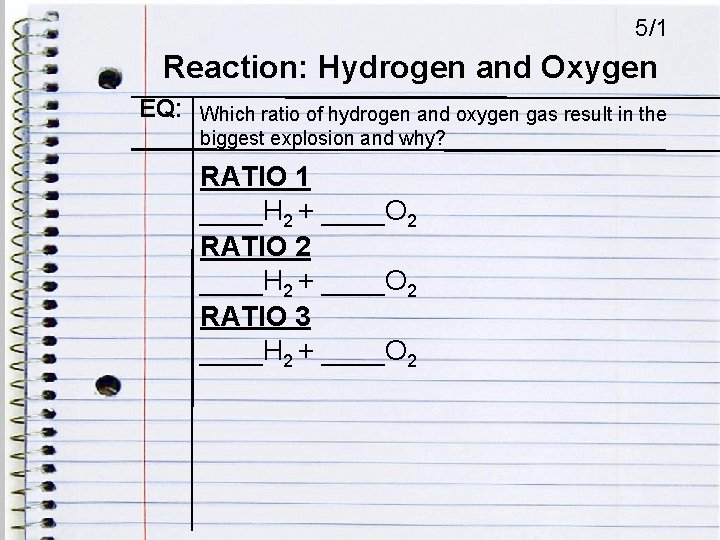
5/1 Reaction: Hydrogen and Oxygen EQ: Which ratio of hydrogen and oxygen gas result in the biggest explosion and why? RATIO 1 ____H 2 + ____O 2 RATIO 2 ____H 2 + ____O 2 RATIO 3 ____H 2 + ____O 2
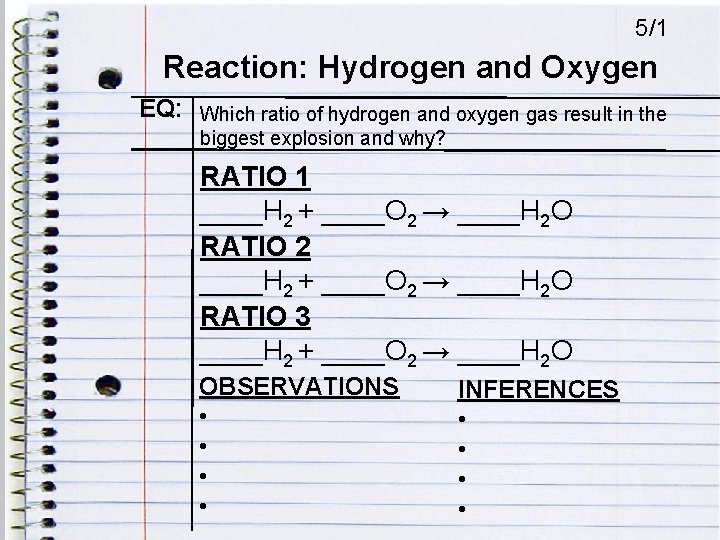
5/1 Reaction: Hydrogen and Oxygen EQ: Which ratio of hydrogen and oxygen gas result in the biggest explosion and why? RATIO 1 ____H 2 + ____O 2 → ____H 2 O RATIO 2 ____H 2 + ____O 2 → ____H 2 O RATIO 3 ____H 2 + ____O 2 → ____H 2 O OBSERVATIONS • • INFERENCES • •
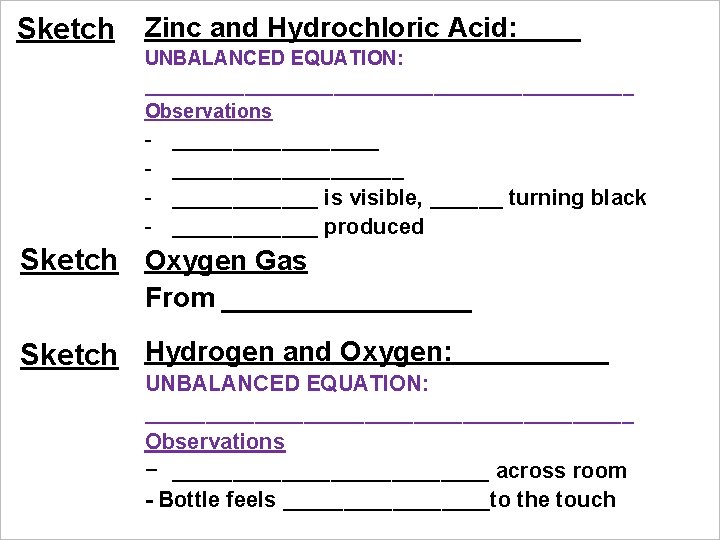
Sketch Zinc and Hydrochloric Acid: UNBALANCED EQUATION: ______________________ Observations - ___________________ is visible, ______ turning black ______ produced Sketch Oxygen Gas From ________ Sketch Hydrogen and Oxygen: UNBALANCED EQUATION: ____________________ Observations − _____________ across room - Bottle feels _________to the touch
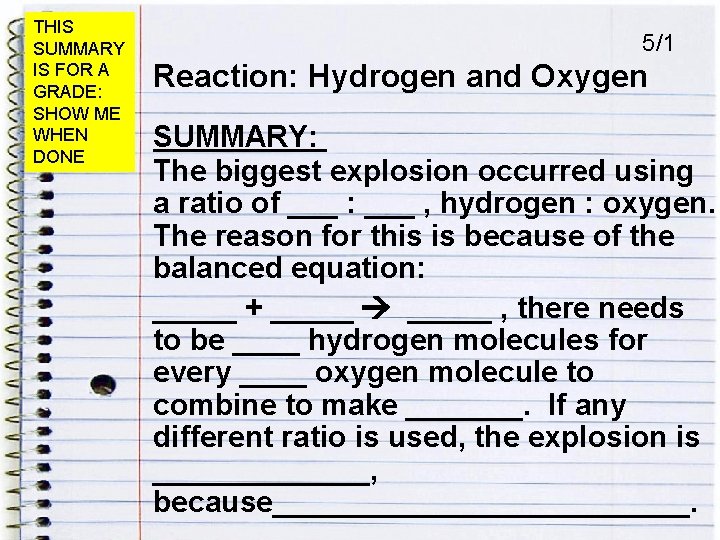
THIS SUMMARY IS FOR A GRADE: SHOW ME WHEN DONE 5/1 Reaction: Hydrogen and Oxygen SUMMARY: The biggest explosion occurred using a ratio of ___ : ___ , hydrogen : oxygen. The reason for this is because of the balanced equation: _____ + _____ , there needs to be ____ hydrogen molecules for every ____ oxygen molecule to combine to make _______. If any different ratio is used, the explosion is _______, because_____________.
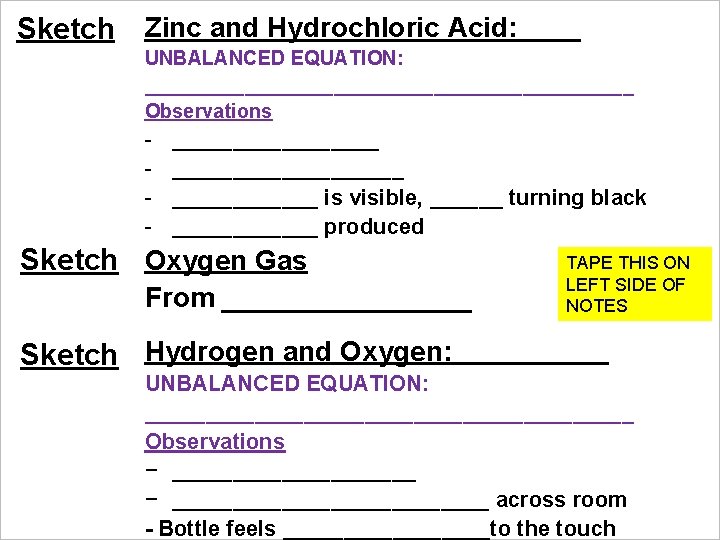
Sketch Zinc and Hydrochloric Acid: UNBALANCED EQUATION: ______________________ Observations - ___________________ is visible, ______ turning black ______ produced Sketch Oxygen Gas From ________ Sketch Hydrogen and Oxygen: TAPE THIS ON LEFT SIDE OF NOTES UNBALANCED EQUATION: ____________________ Observations − __________________________ across room - Bottle feels _________to the touch

Sketch Zinc and Hydrochloric Acid: EQUATION: Zn (s) + 2 HCl (aq) Zn. Cl 2 (aq) + H 2 (g) - Immediate reaction, vigorous bubbles - Gas is visible, Zn turning black, heat produced Sketch Oxygen Gas From Oxygen Tank Hydrogen and Oxygen: Sketch EQUATION: 2 H 2 + O 2 2 H 2 O - Loud explosion, bottle shot across room - Bottle feels warm to the touch

Types of Chemical Reactions Webquest COMPLETE: Introduction Reading CHECK OUT: Chromebook GO TO: Suggested Websites COMPLETE: Pages 2 -4 of Assignment TIME: 34 MINUTES (Due Today) WHEN DONE: Turn into class box
- Slides: 14