Unit 6 Chemical Nomenclature Ionic Compounds with Transition












- Slides: 22

Unit 6: Chemical Nomenclature - Ionic Compounds with Transition Metals and Polyatomic Ions

Write the formula for these: 1. Sodium Chloride 2. Calcium nitride 3. Barium oxide 4. Iron (III) oxide 5. Sodium phosphate

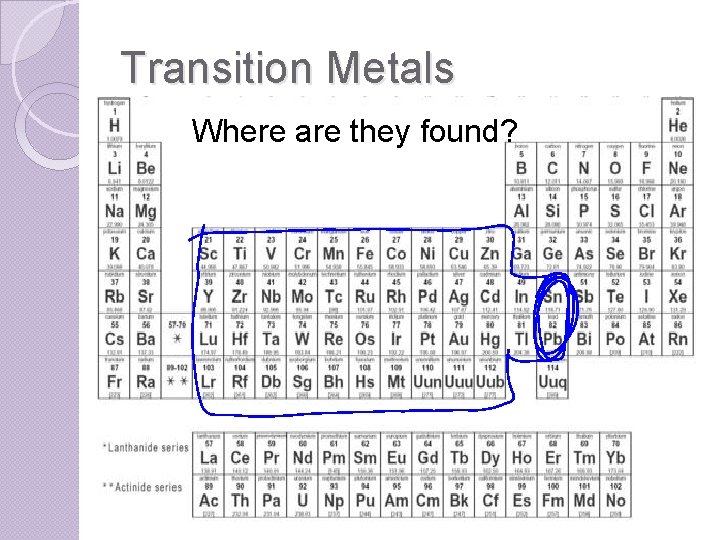
Transition Metals Where are they found?

Why are they called transition? �Have multiple oxidation numbers they transition in between Example: Iron (II) and Iron (III) Fe+2 and Fe+3

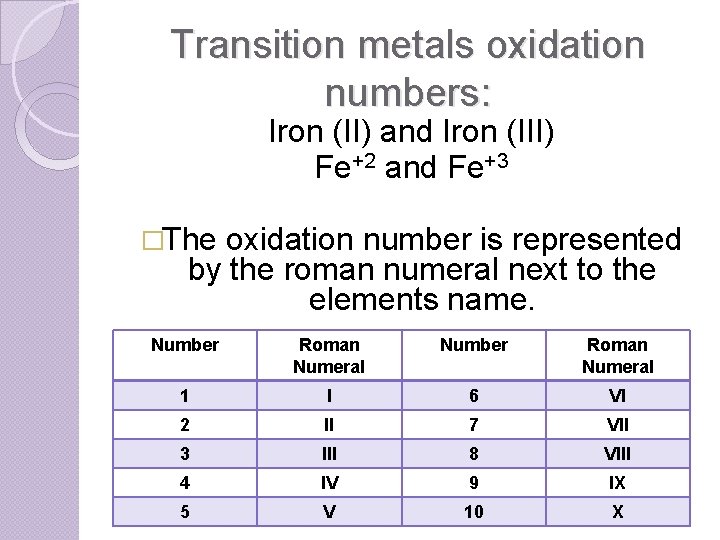
Transition metals oxidation numbers: Iron (II) and Iron (III) Fe+2 and Fe+3 �The oxidation number is represented by the roman numeral next to the elements name. Number Roman Numeral 1 I 6 VI 2 II 7 VII 3 III 8 VIII 4 IV 9 IX 5 V 10 X

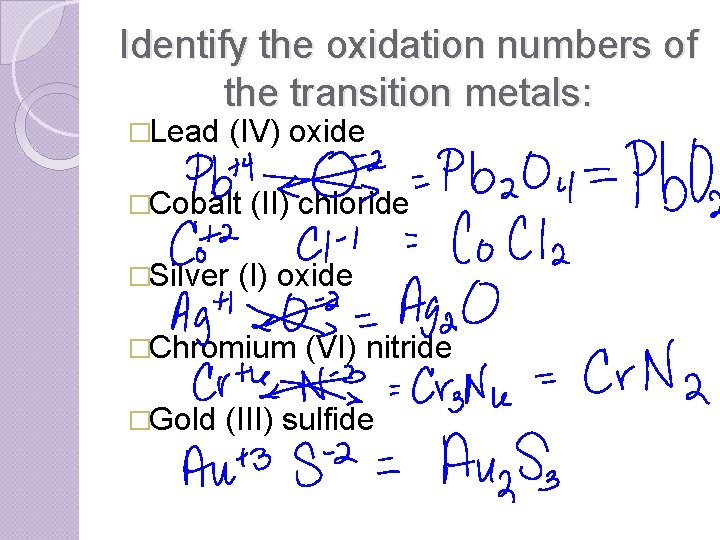
Identify the oxidation numbers of the transition metals: �Lead (IV) oxide �Cobalt �Silver (II) chloride (I) oxide �Chromium �Gold (VI) nitride (III) sulfide

Writing Formulas for Transition Metals: �Manganese (VII) sulfide Cation symbol: _____ Anion symbol: _____

Practice: �Gold (III) Oxide �Titanium �Zinc (IV) sulfide (I) chloride

Writing the names of Ionic Compounds with Transition Metals �Just like you write the names of any ionic compound, but you MUST include the OXIDATION NUMBER of the transition metal. ◦ This is completed using a roman numeral Iron (III) oxide

Identifying the oxidation number �Most of the time it is easily identified, other times it is not. �To get the oxidation numbers you simply undrop and unswap Fe 2 O 3 Iron (III) Oxide • Use the oxidation number of the nonmetal to check your work. • Does it match the periodic table? • If yes, you are good to go • If no, you will need to unsimplify

Example: Mn. O 2 �Begin by unswapping and dropping Mn. O 2 Mn +2 and O -1 What is the oxidation number of oxygen? _____ (according to the periodic table) Does it match (yes or no)? ______ What do we need to do to unsimplify it?

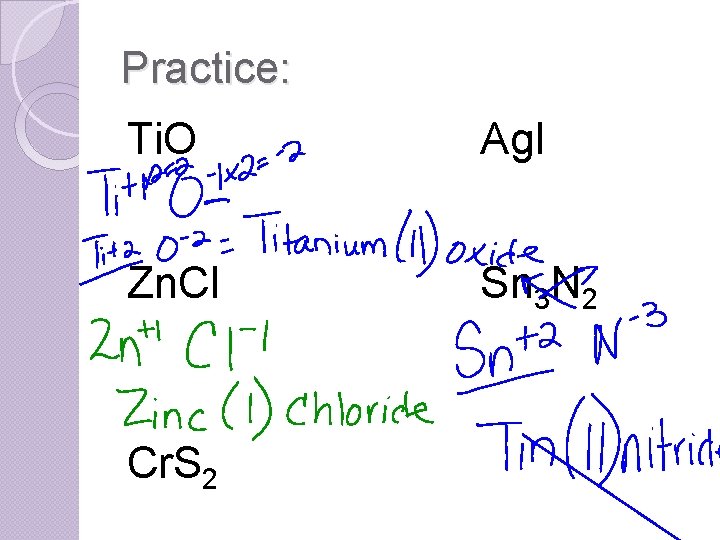
Practice: Ti. O Ag. I Zn. Cl Sn 3 N 2 Cr. S 2

Ionic Compounds with Polyatomic Ions �A polyatomic ion is simply a compound with a charge. ◦ Poly = many or more than 1 ◦ Atomic = atoms ◦ Ion = charged atom(s)

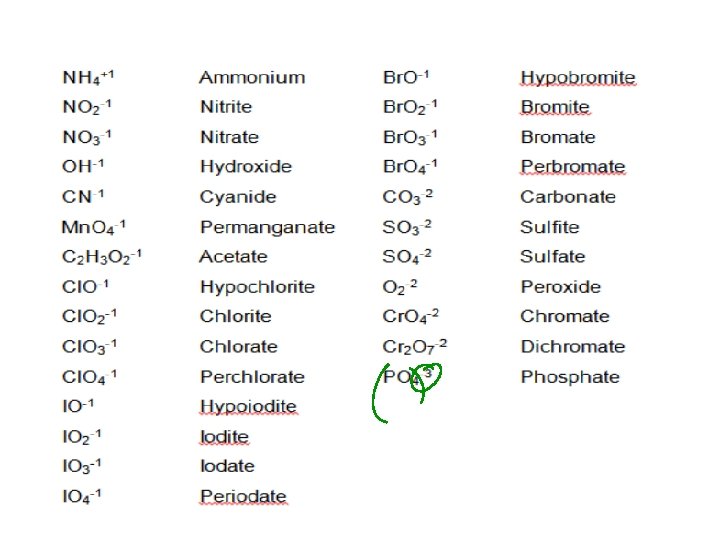
Where can I find these? �Take a look at the back of your periodic table… ◦ Those are you polyatomic ions


Looking at your polyatomic ions what do you notice?

Polyatomics �Are treated as single units instead of individual elements �The charge of the ion is the oxidation number �We cage the beasts �Polyatomic ions keep their names �The rest of the ionic compound naming rules apply

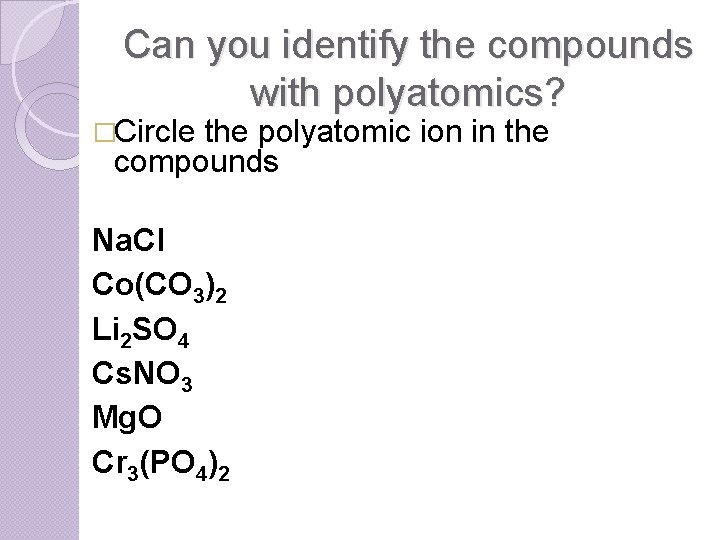
Can you identify the compounds with polyatomics? �Circle the polyatomic ion in the compounds Na. Cl Co(CO 3)2 Li 2 SO 4 Cs. NO 3 Mg. O Cr 3(PO 4)2

Naming compounds with polyatomic ions �Name the cation *If there is a transition metal make sure to include its oxidation number �Name the anion *If it is NOT a polyatomic don’t forget to change the ending to –ide.

Example: �Na 2(CO 3) �Mg 3(PO 4)2 �Pb(NO 3)2

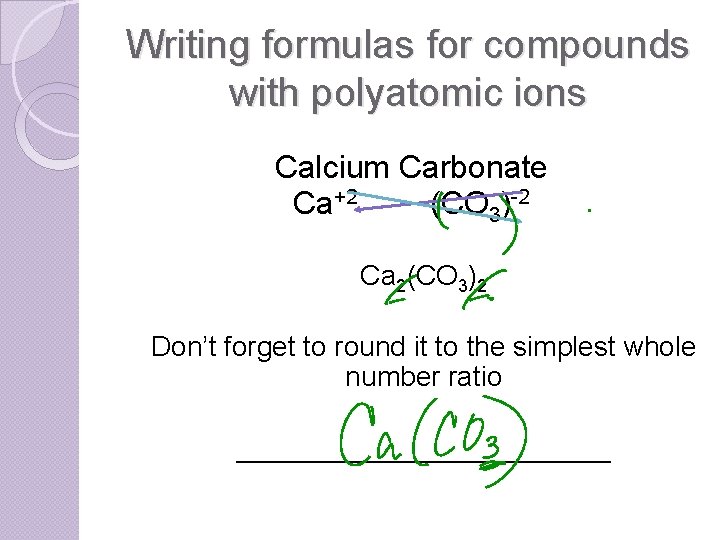
Writing formulas for compounds with polyatomic ions Calcium Carbonate Ca+2 (CO 3)-2 Ca 2(CO 3)2 Don’t forget to round it to the simplest whole number ratio ____________

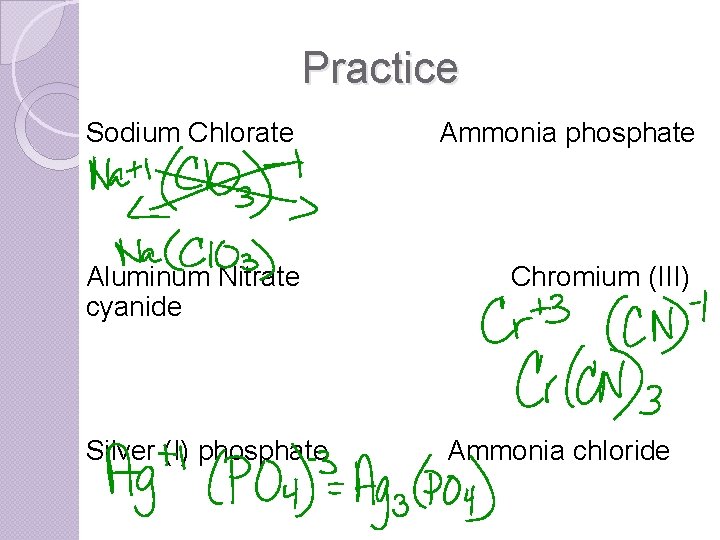
Practice Sodium Chlorate Ammonia phosphate Aluminum Nitrate cyanide Chromium (III) Silver (I) phosphate Ammonia chloride