Unit 6 Chemical Bonding and Intermolecular Forces Chemistry

Unit 6: Chemical Bonding and Intermolecular Forces Chemistry I

7. 1 Ions: Goals 1 -5 REVIEW! • Valence Electrons – Electrons that occupy the highest energy level of an atom. – The electrons in the outermost orbitals. • Determining the Number of Valence Electrons – All representative elements have a potential for 8 valence electrons • 2 in the s • 6 in the p orbitals – The number of valence electrons is the Group Number. • Octet Rule – All representative elements will gain or lose electrons to form an octet (8) of valence electrons. – To achieve the electron configuration of a Noble Gas
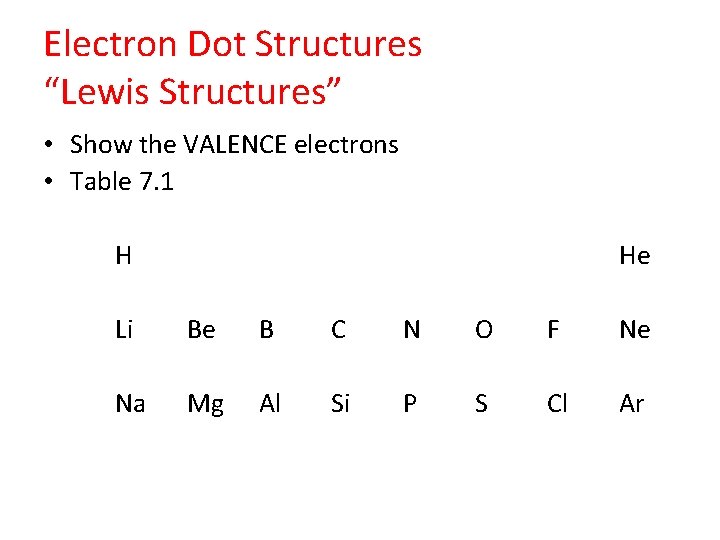
Electron Dot Structures “Lewis Structures” • Show the VALENCE electrons • Table 7. 1 H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar
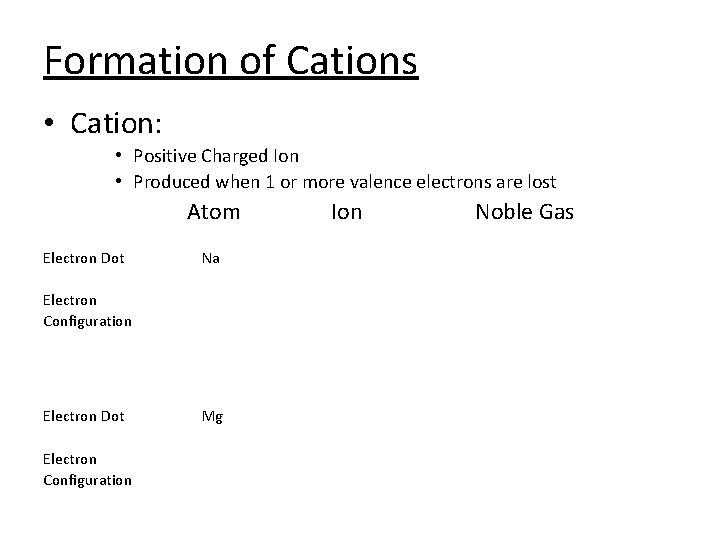
Formation of Cations • Cation: • Positive Charged Ion • Produced when 1 or more valence electrons are lost Atom Electron Dot Na Electron Configuration Electron Dot Electron Configuration Mg Ion Noble Gas
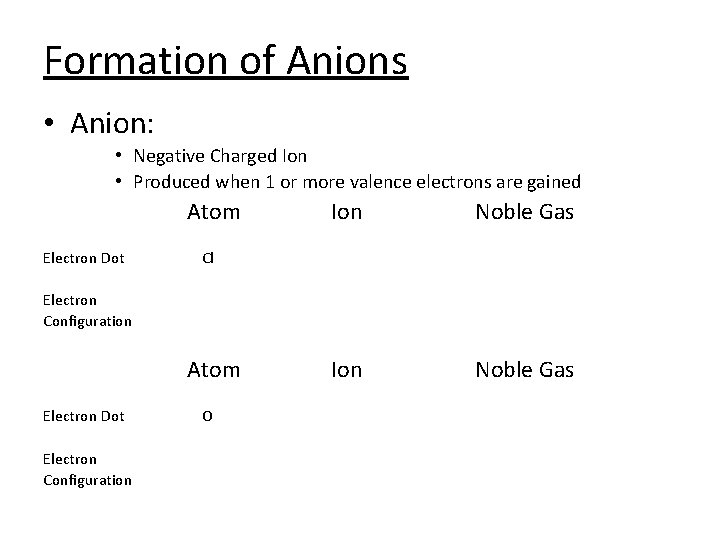
Formation of Anions • Anion: • Negative Charged Ion • Produced when 1 or more valence electrons are gained Atom Electron Dot Ion Noble Gas Cl Electron Configuration Atom Electron Dot Electron Configuration O
7. 2 Ionic Bonds & Ionic Compounds • Ionic Compound • The combination of Anions and Cations. • Attracted to each other by electrical forces (opposites attract). • Although they are made of ions, ionic compounds are electrically neutral Total (+ Charge) = Total (- Charge)
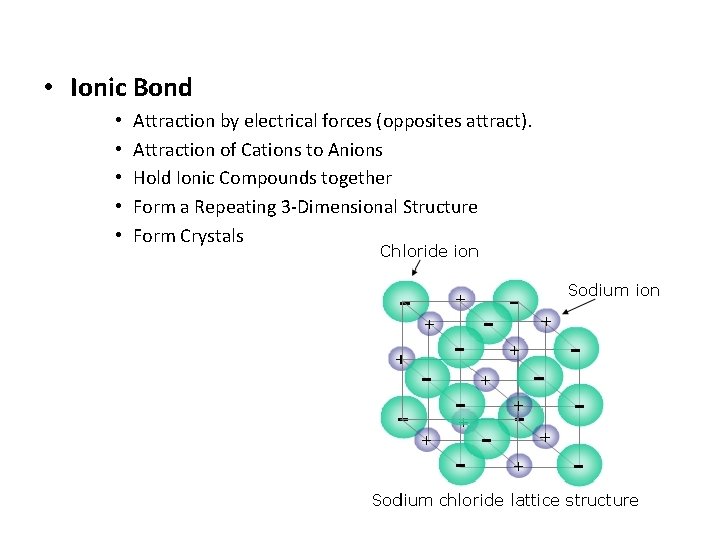
• Ionic Bond • • • Attraction by electrical forces (opposites attract). Attraction of Cations to Anions Hold Ionic Compounds together Form a Repeating 3 -Dimensional Structure Form Crystals
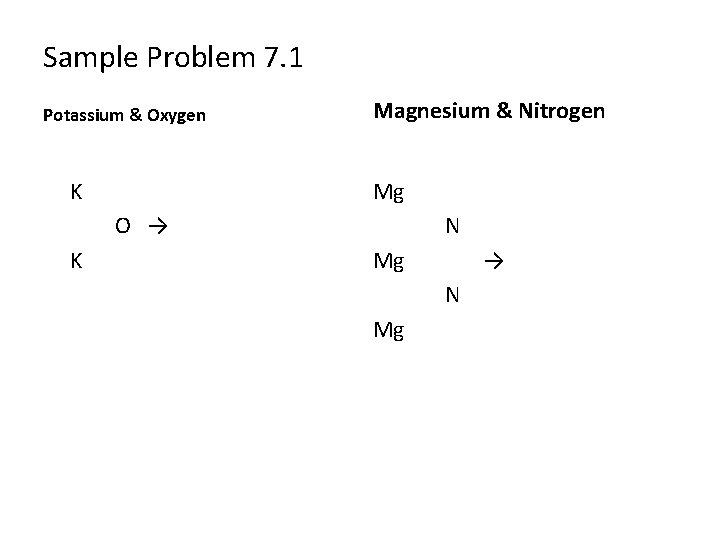
Sample Problem 7. 1 Potassium & Oxygen K Magnesium & Nitrogen Mg O → K N Mg → N Mg
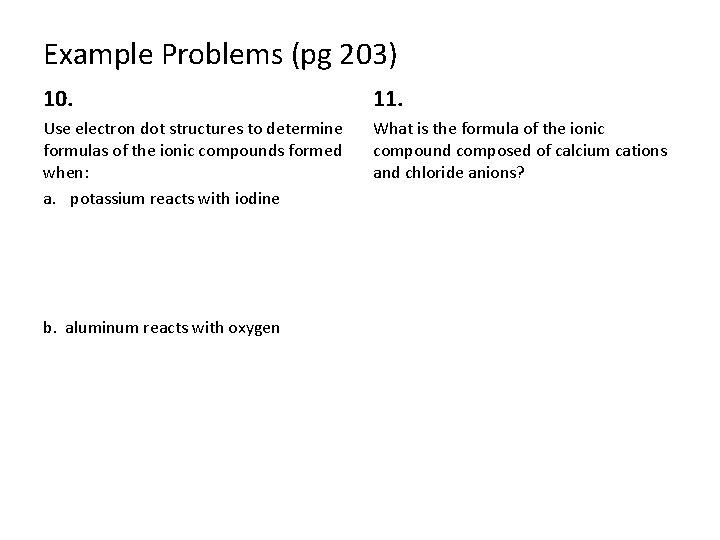
Example Problems (pg 203) 10. 11. Use electron dot structures to determine formulas of the ionic compounds formed when: a. potassium reacts with iodine What is the formula of the ionic compound composed of calcium cations and chloride anions? b. aluminum reacts with oxygen

Properties of Ionic Compounds 1. Form solid crystal structures based on number and size of Anions to Cations Crystal Shape? 2. Generally have HIGH melting points 3. Can conduct an electric current when melted or dissolved in water.
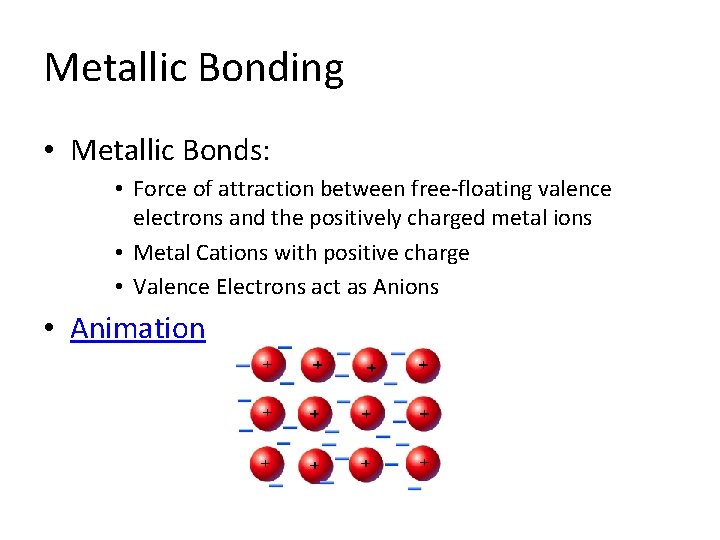
Metallic Bonding • Metallic Bonds: • Force of attraction between free-floating valence electrons and the positively charged metal ions • Metal Cations with positive charge • Valence Electrons act as Anions • Animation
Properties of Metals • Malleable and Ductile because of the Metallic Bonds. – The valence electrons and Cations can move about each other – Allow the overall structure to bend or give Malleable: Can be hammered or pressed into shapes - BENT Ductile: Can be drawn into wires - STRETCHED • Conduct Electricity because of the Metallic Bonds – Valence Electrons are able to move around or flow • Alloys – Metallic Bonding with more than one type of metal cation. – Examples: • Bronze: Copper & Tin • Brass: Copper & Zinc • Steel: Iron & Carbon
Chapter 8: Goals 6 -13 8. 1 Molecules and Molecular Compounds • Covalent Bond: • A sharing of electrons • Usually occurs between 2 or more NON-METALS • Molecule • A group of atoms joined together by covalent bonds • 2 types: 1. Molecular Compounds: 2 or more Elements Covalently bonded Example: Water H 2 O 2. Diatomic Molecule: Element in Molecule form Examples: Oxygen O 2 Hydrogen Nitrogen Halogens
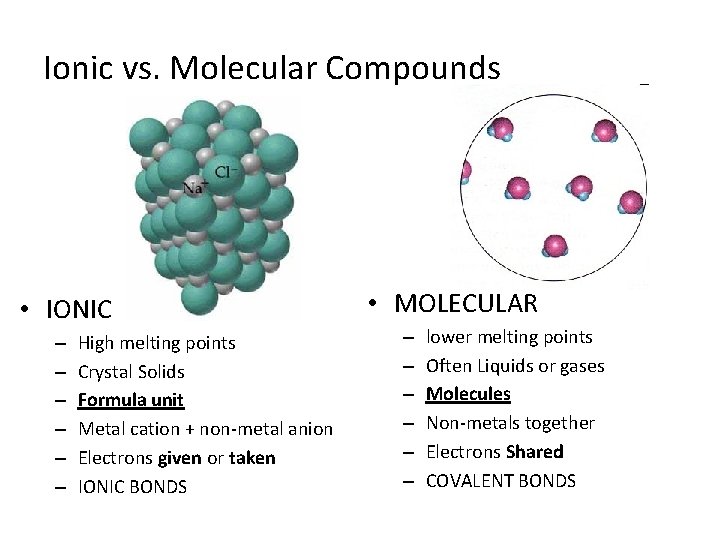
Ionic vs. Molecular Compounds • IONIC – – – High melting points Crystal Solids Formula unit Metal cation + non-metal anion Electrons given or taken IONIC BONDS • MOLECULAR – – – lower melting points Often Liquids or gases Molecules Non-metals together Electrons Shared COVALENT BONDS
8. 2 Nature of Covalent Bonding Octet rule in Covalent Bonding • Octet Rule – shared electrons count for each atoms “octet” – Hydrogen is an exception (only 2 e-) • Single Covalent Bond – one pair (2 e-) are shared Ex) H 2 • Double Covalent Bond – two pairs (4 e-) are shared: Considered 1 bond Ex) O 2 • Triple Covalent Bond – three pairs (6 e-) are shared: Considered 1 bond Ex) N 2
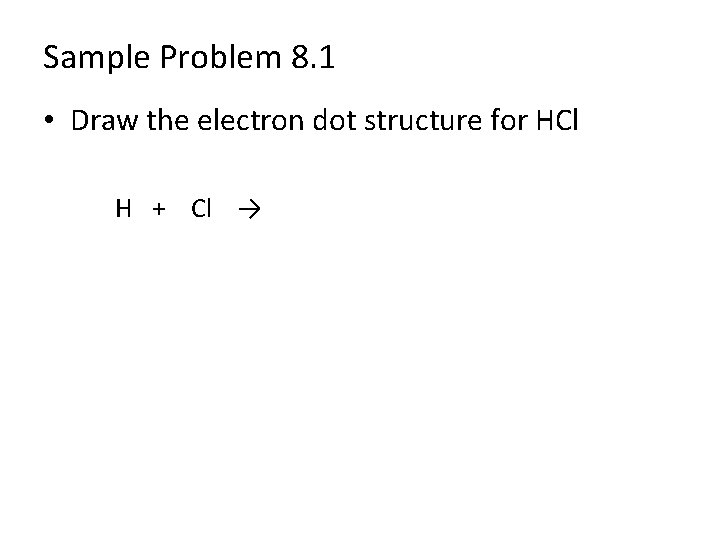
Sample Problem 8. 1 • Draw the electron dot structure for HCl H + Cl →
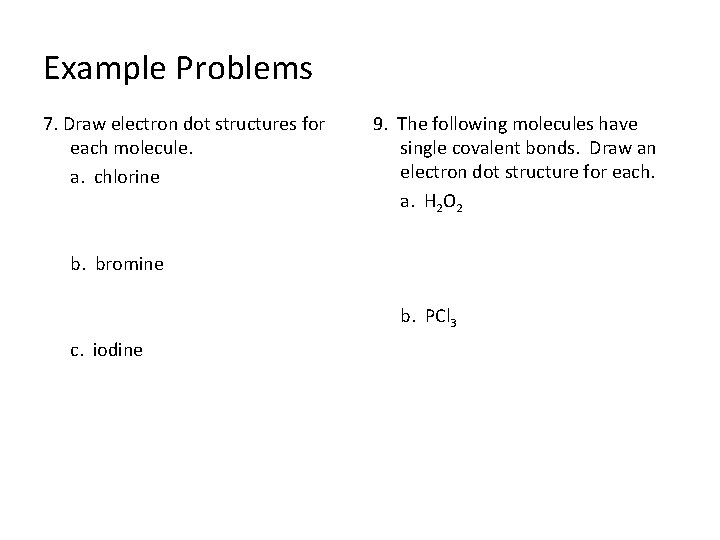
Example Problems 7. Draw electron dot structures for each molecule. a. chlorine 9. The following molecules have single covalent bonds. Draw an electron dot structure for each. a. H 2 O 2 b. bromine b. PCl 3 c. iodine
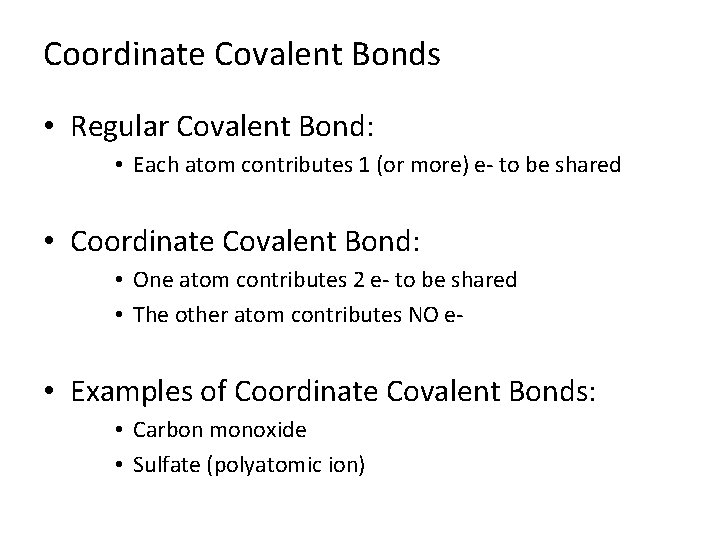
Coordinate Covalent Bonds • Regular Covalent Bond: • Each atom contributes 1 (or more) e- to be shared • Coordinate Covalent Bond: • One atom contributes 2 e- to be shared • The other atom contributes NO e- • Examples of Coordinate Covalent Bonds: • Carbon monoxide • Sulfate (polyatomic ion)
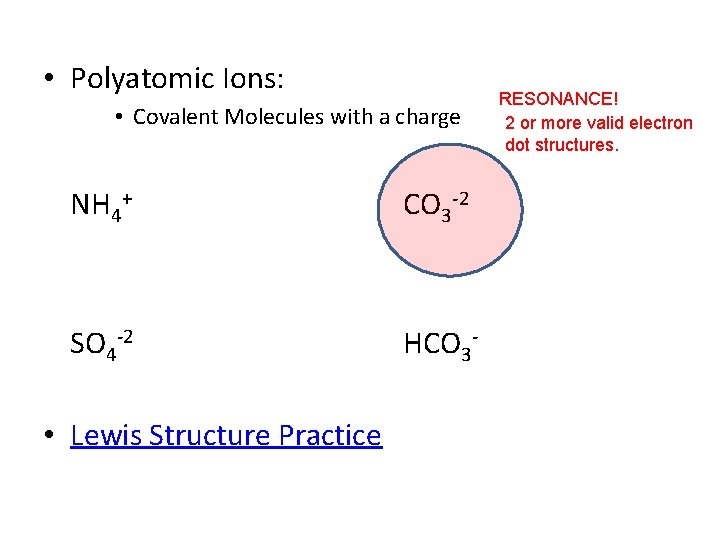
• Polyatomic Ions: • Covalent Molecules with a charge NH 4+ CO 3 -2 SO 4 -2 HCO 3 - • Lewis Structure Practice RESONANCE! 2 or more valid electron dot structures.
VSEPR Theory: Molecule Shape Valence Shell Electron Pair Repulsion • The shapes of molecules depends on: • the number of covalent bonds • the types of covalent bonds (single, double, etc. ), • the number of unshared electrons in the central atom. § Bonded Pairs and Lone Pairs REPELL each other! • Demonstration
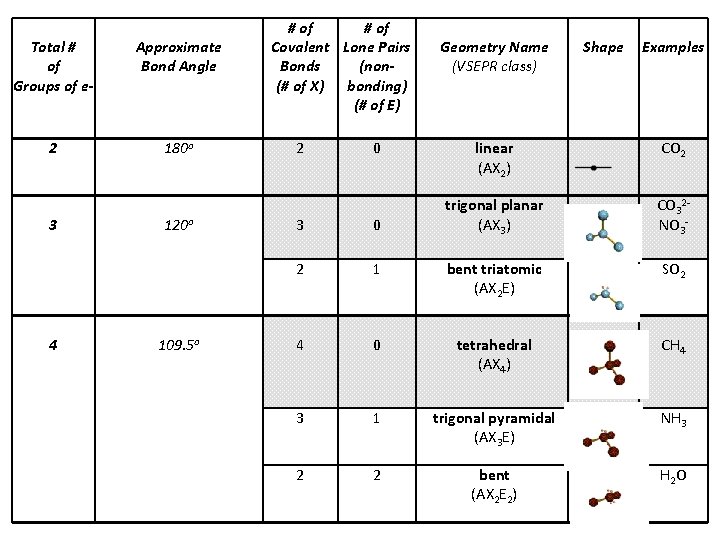
Total # of Groups of e- Approximate Bond Angle 2 180 o 3 4 120 o 109. 5 o # of Covalent Lone Pairs Bonds (non(# of X) bonding) (# of E) 2 0 Geometry Name (VSEPR class) Shape Examples linear (AX 2) CO 2 trigonal planar (AX 3) CO 32 NO 3 - 3 0 2 1 bent triatomic (AX 2 E) SO 2 4 0 tetrahedral (AX 4) CH 4 3 1 trigonal pyramidal (AX 3 E) NH 3 2 2 bent (AX 2 E 2) H 2 O
VSEPR Shape Information • Double and Triple bonds count as just 1 bond each • Names correspond to a “central” atom • Some molecules have multiple “central” atoms. • Example: H 2 O 2 – Double bent
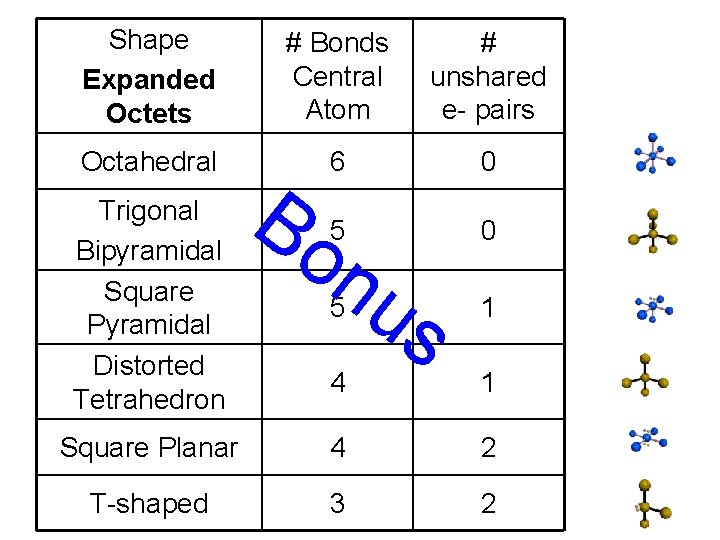
Shape Expanded Octets # Bonds Central Atom # unshared e- pairs Octahedral 6 0 5 0 Trigonal Bipyramidal Square Pyramidal Distorted Tetrahedron Bo nu 5 s 1 4 1 Square Planar 4 2 T-shaped 3 2
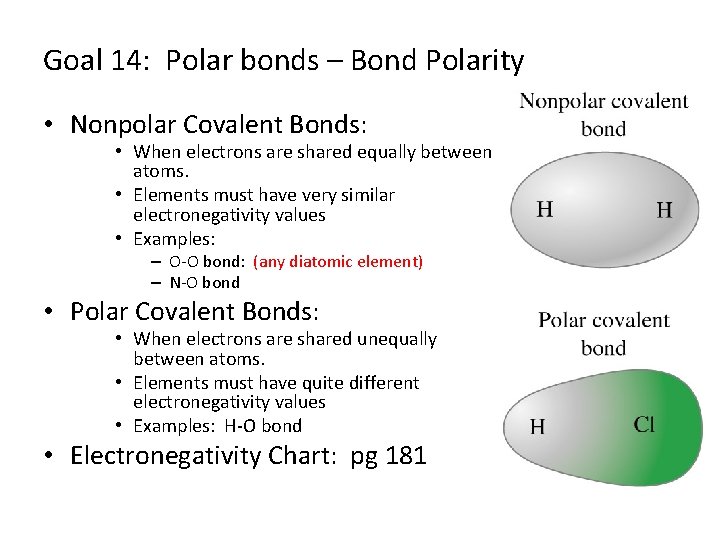
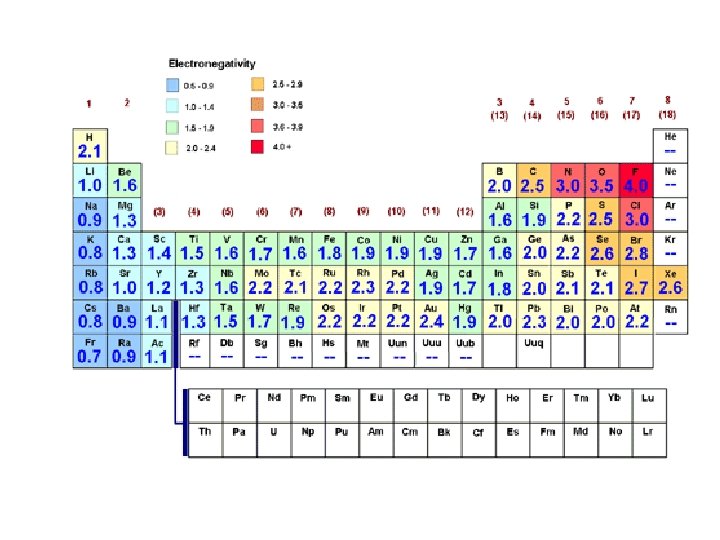
Goal 14: Polar bonds – Bond Polarity • Nonpolar Covalent Bonds: • When electrons are shared equally between atoms. • Elements must have very similar electronegativity values • Examples: – O-O bond: (any diatomic element) – N-O bond • Polar Covalent Bonds: • When electrons are shared unequally between atoms. • Elements must have quite different electronegativity values • Examples: H-O bond • Electronegativity Chart: pg 181
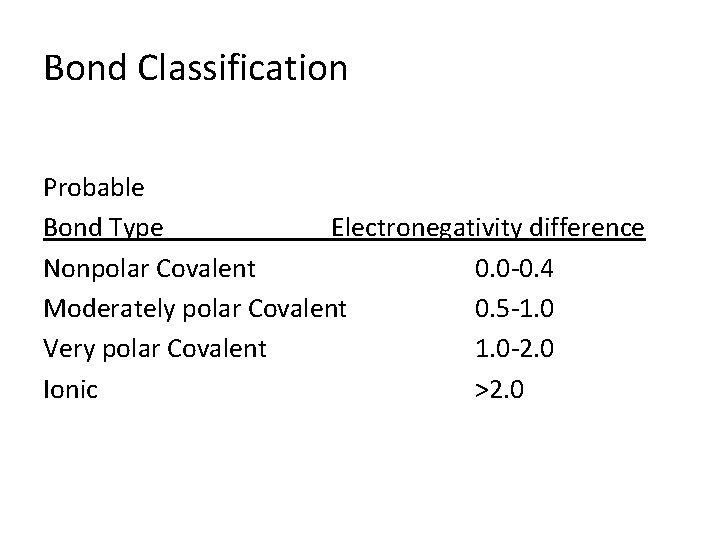
Bond Classification Probable Bond Type Electronegativity difference Nonpolar Covalent 0. 0 -0. 4 Moderately polar Covalent 0. 5 -1. 0 Very polar Covalent 1. 0 -2. 0 Ionic >2. 0
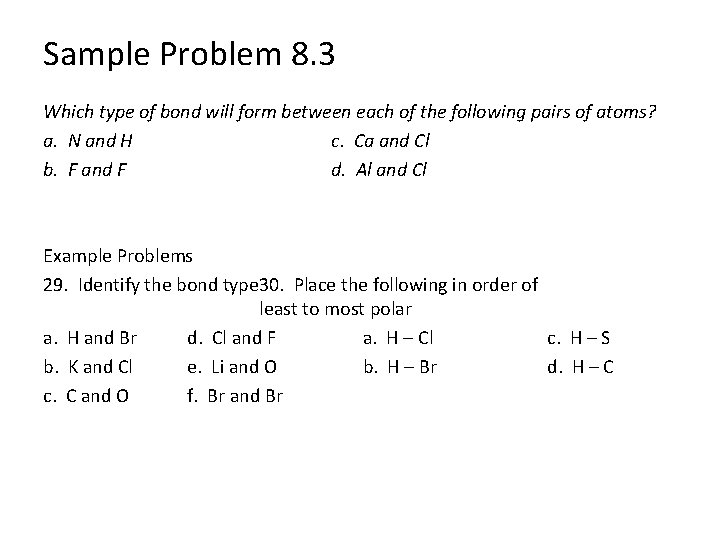
Sample Problem 8. 3 Which type of bond will form between each of the following pairs of atoms? a. N and H c. Ca and Cl b. F and F d. Al and Cl Example Problems 29. Identify the bond type 30. Place the following in order of least to most polar a. H and Br d. Cl and F a. H – Cl c. H – S b. K and Cl e. Li and O b. H – Br d. H – C c. C and O f. Br and Br
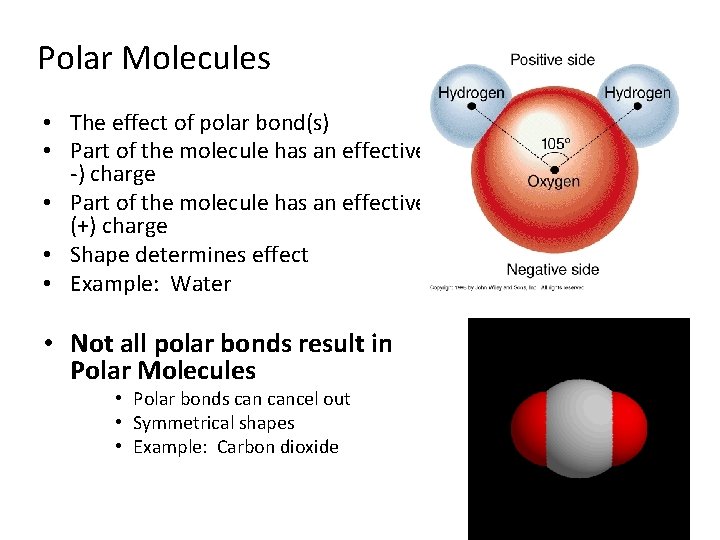
Polar Molecules • The effect of polar bond(s) • Part of the molecule has an effective ( -) charge • Part of the molecule has an effective (+) charge • Shape determines effect • Example: Water • Not all polar bonds result in Polar Molecules • Polar bonds cancel out • Symmetrical shapes • Example: Carbon dioxide
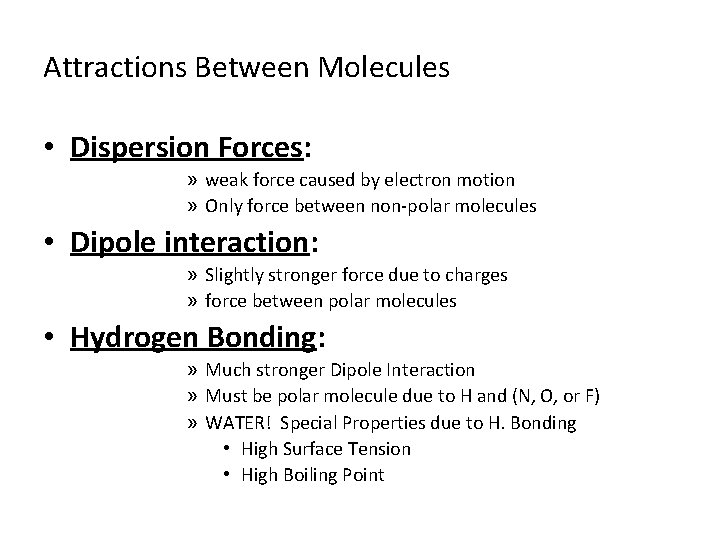
Attractions Between Molecules • Dispersion Forces: » weak force caused by electron motion » Only force between non-polar molecules • Dipole interaction: » Slightly stronger force due to charges » force between polar molecules • Hydrogen Bonding: » Much stronger Dipole Interaction » Must be polar molecule due to H and (N, O, or F) » WATER! Special Properties due to H. Bonding • High Surface Tension • High Boiling Point
- Slides: 29