UNIT 6 BALANCING CHEMICAL REACTIONS AND REACTION TYPES

UNIT 6: BALANCING CHEMICAL REACTIONS AND REACTION TYPES

Key Objectives ØIdentify when a reaction occurs ØIdentify why reactions occur ØIdentify the parts of a reaction (see ws) ØIdentify the different types of reactions ØBe able to balance reactions

A Reaction Has Occurred When… ØGas is formed ØEnergy ØColor is exchanged changes ØPrecipitate
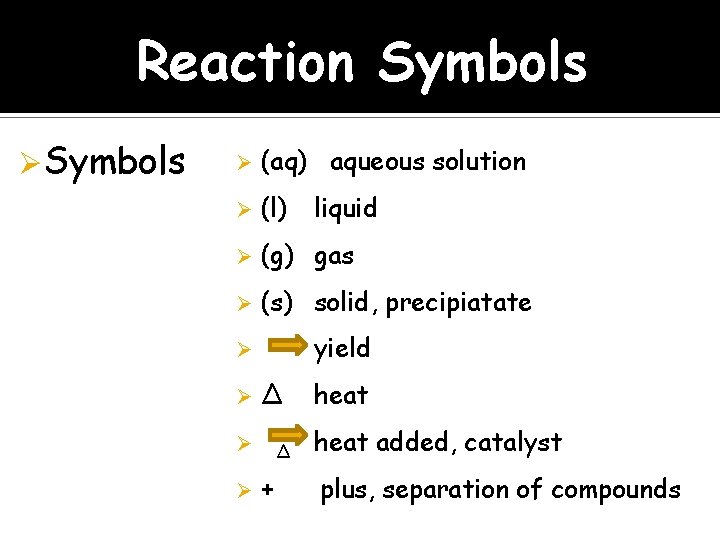
Reaction Symbols Ø (aq) aqueous solution Ø (l) Ø (g) gas Ø (s) solid, precipiatate yield Ø Ø ∆ Ø Ø liquid ∆ + heat added, catalyst plus, separation of compounds
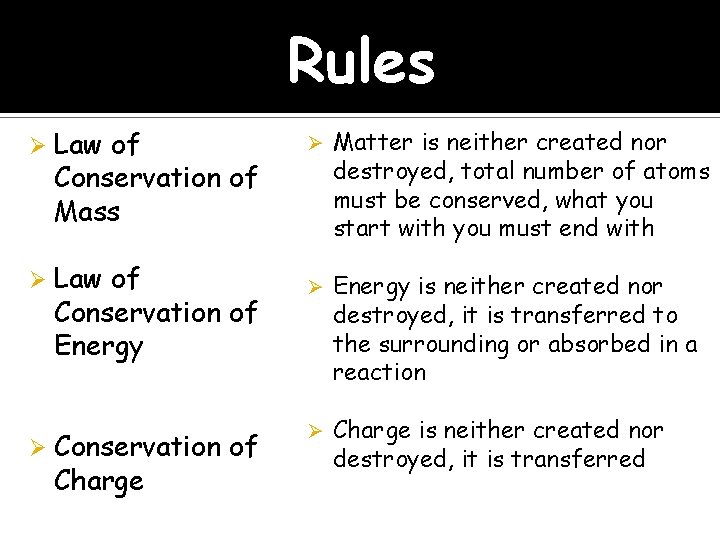
Rules Ø Law of Conservation of Mass Ø Law of Conservation of Energy Ø Conservation of Charge Ø Matter is neither created nor destroyed, total number of atoms must be conserved, what you start with you must end with Ø Energy is neither created nor destroyed, it is transferred to the surrounding or absorbed in a reaction Ø Charge is neither created nor destroyed, it is transferred
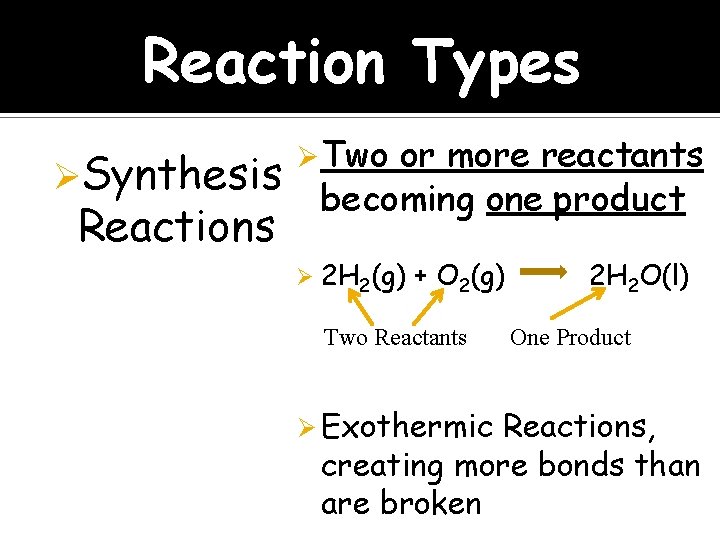
Reaction Types ØSynthesis Ø Two or more reactants becoming one product Reactions Ø 2 H 2(g) + O 2(g) Two Reactants Ø Exothermic 2 H 2 O(l) One Product Reactions, creating more bonds than are broken
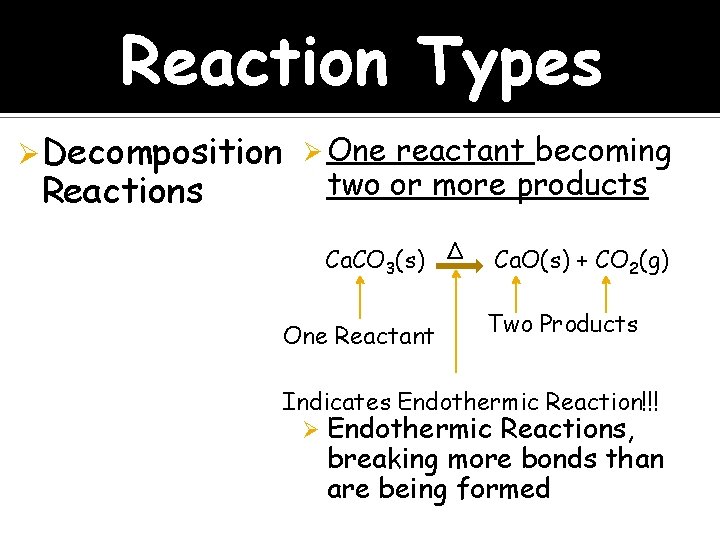
Reaction Types Ø Decomposition Ø One reactant becoming Reactions two or more products Ca. CO 3(s) ∆ One Reactant Ca. O(s) + CO 2(g) Two Products Indicates Endothermic Reaction!!! Ø Endothermic Reactions, breaking more bonds than are being formed
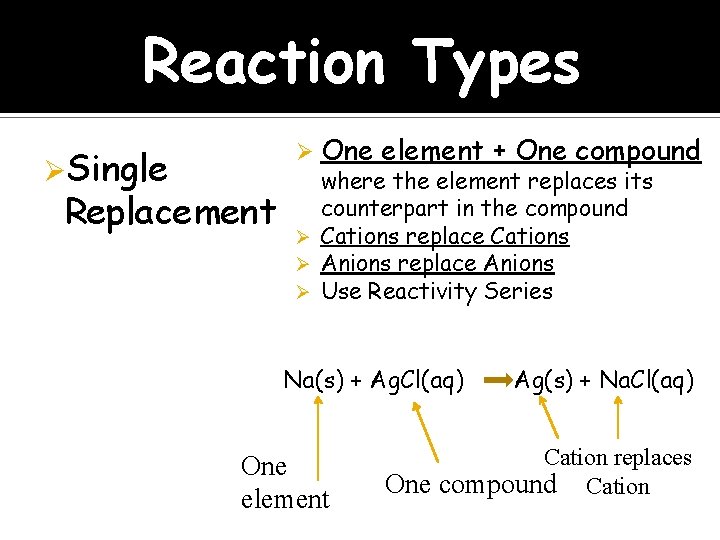
Reaction Types ØSingle Ø Replacement One element + One compound where the element replaces its counterpart in the compound Ø Cations replace Cations Ø Anions replace Anions Ø Use Reactivity Series Na(s) + Ag. Cl(aq) One element Ag(s) + Na. Cl(aq) Cation replaces One compound Cation
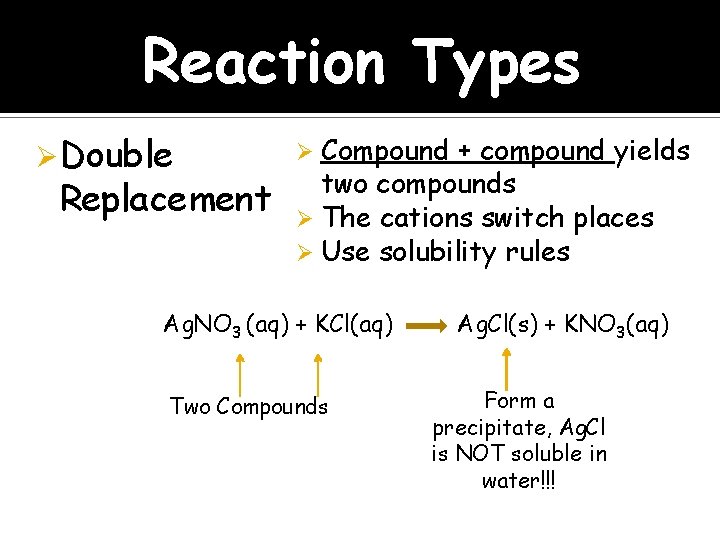
Reaction Types Ø Double Replacement Compound + compound yields two compounds Ø The cations switch places Ø Use solubility rules Ø Ag. NO 3 (aq) + KCl(aq) Two Compounds Ag. Cl(s) + KNO 3(aq) Form a precipitate, Ag. Cl is NOT soluble in water!!!
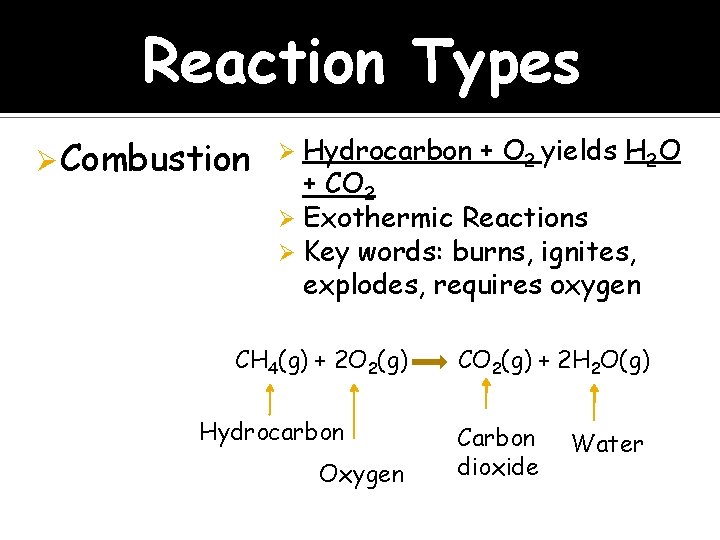
Reaction Types Ø Combustion Hydrocarbon + O 2 yields H 2 O + CO 2 Ø Exothermic Reactions Ø Key words: burns, ignites, explodes, requires oxygen Ø CH 4(g) + 2 O 2(g) Hydrocarbon Oxygen CO 2(g) + 2 H 2 O(g) Carbon dioxide Water
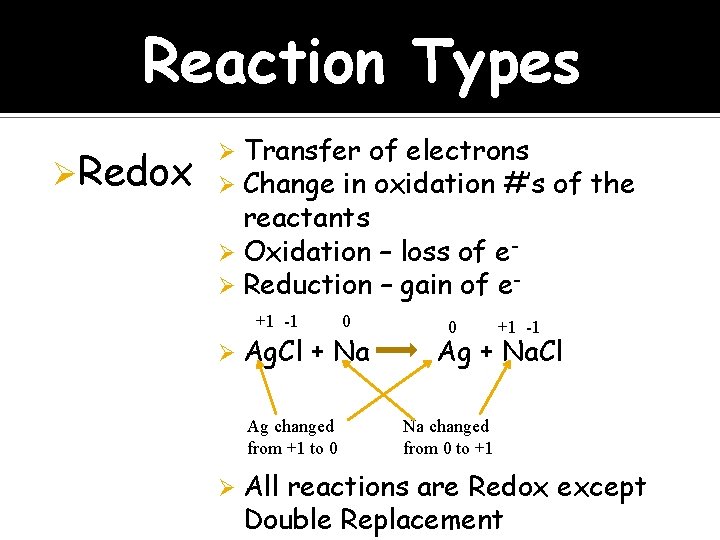
Reaction Types ØRedox Transfer of electrons Change in oxidation #’s of the reactants Ø Oxidation – loss of eØ Reduction – gain of eØ Ø +1 -1 Ø Ag. Cl + Na Ag changed from +1 to 0 Ø 0 0 +1 -1 Ag + Na. Cl Na changed from 0 to +1 All reactions are Redox except Double Replacement
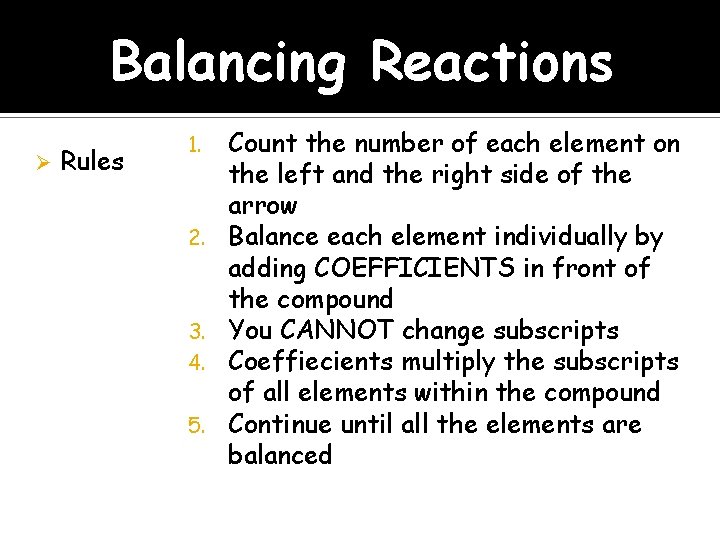
Balancing Reactions Ø Rules 1. 2. 3. 4. 5. Count the number of each element on the left and the right side of the arrow Balance each element individually by adding COEFFICIENTS in front of the compound You CANNOT change subscripts Coeffiecients multiply the subscripts of all elements within the compound Continue until all the elements are balanced
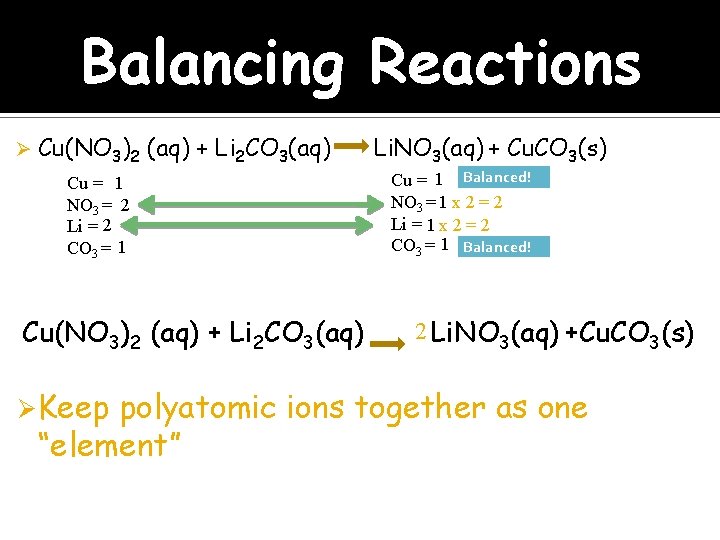
Balancing Reactions Ø Cu(NO 3)2 (aq) + Li 2 CO 3(aq) Cu = 1 NO 3 = 2 Li = 2 CO 3 = 1 Cu(NO 3)2 (aq) + Li 2 CO 3(aq) Ø Keep Li. NO 3(aq) + Cu. CO 3(s) Cu = 1 Balanced! NO 3 = 1 x 2 = 2 Li = 1 x 2 = 2 CO 3 = 1 Balanced! 2 Li. NO 3(aq) +Cu. CO 3(s) polyatomic ions together as one “element”
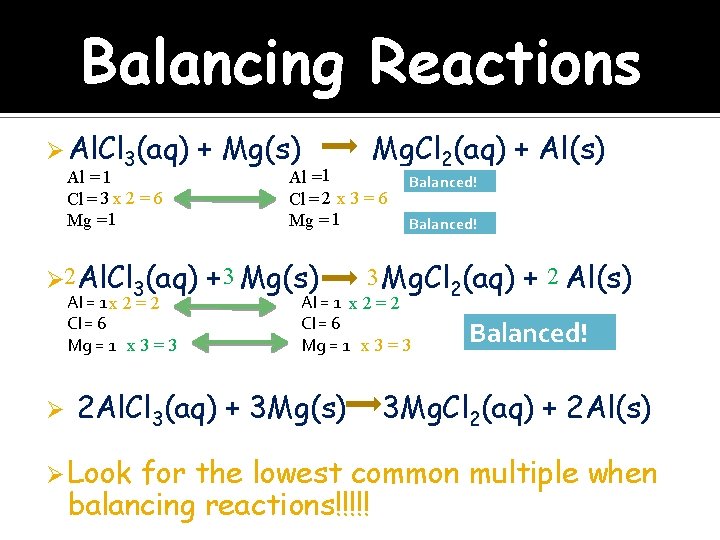
Balancing Reactions Ø Al. Cl 3(aq) Al = 1 Cl = 3 x 2 = 6 Mg = 1 + Mg(s) Ø 2 Al. Cl 3(aq) Al = 1 x 2 = 2 Cl = 6 x 2 =6 Mg = 1 x 3 = 3 Ø Mg. Cl 2(aq) + Al(s) Al =1 Cl = 2 x 3 = 6 Mg = 1 + 3 Mg(s) Balanced! 3 Mg. Cl 2(aq) Al = 1 x 2 = 2 Cl = 6 x 3 = 6 Mg = 1 x 3 = 3 2 Al. Cl 3(aq) + 3 Mg(s) Ø Look Balanced! + 2 Al(s) Balanced! 3 Mg. Cl 2(aq) + 2 Al(s) for the lowest common multiple when balancing reactions!!!!!

Balancing Reactions Ø Combustion Reactions Ø Balance Carbon 1 st Ø Balance Hydrogen 2 nd Ø Count all your Oxygen atoms on both the left and the right side of the arrow Ø If the right side of the arrow contains an odd number of oxygen atoms, multiply the entire equation by two EXCEPT OXYGEN Ø Balance the Oxygen atoms last
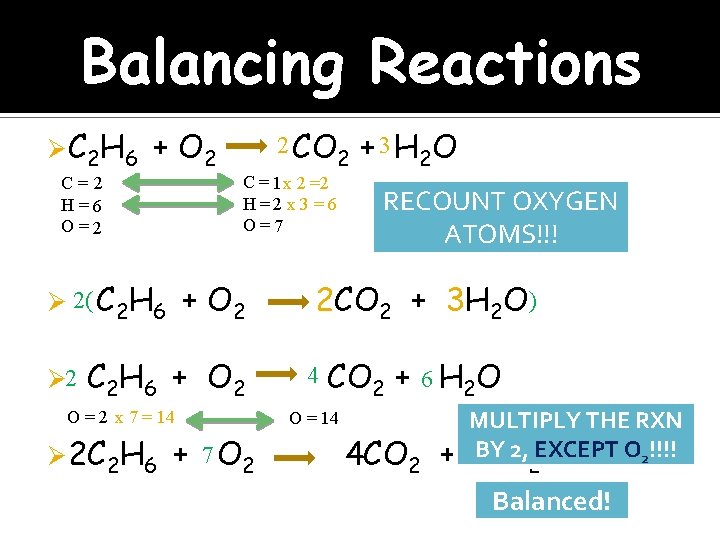
Balancing Reactions Ø C 2 H 6 + O 2 Ø 2( C 2 H 6 + O 2 C 2 H 6 + O 2 O = 2 x 7 = 14 Ø 2 C 2 H 6 2 C = 1 x 2 =2 H =2 x 3 = 6 O =7 C= 2 H=6 O=2 Ø 2 2 CO + 7 O 2 + 3 H 2 O RECOUNT OXYGEN ATOMS!!! 2 CO 2 + 3 H 2 O ) 4 CO 2 + 6 H 2 O O = 14 4 CO 2 + MULTIPLY THE RXN BY 2, EXCEPT O 2!!!! 6 H O 2 Balanced!
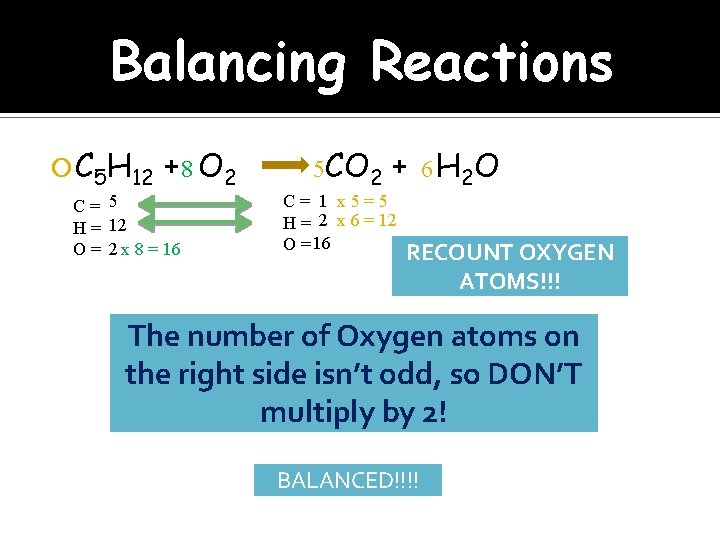
Balancing Reactions C 5 H 12 + 8 O 2 C= 5 H = 12 O = 2 x 8 = 16 5 CO 2 + 6 H 2 O C= 1 x 5=5 H = 2 x 6 = 12 O = 16 RECOUNT OXYGEN ATOMS!!! The number of Oxygen atoms on the right side isn’t odd, so DON’T multiply by 2! BALANCED!!!!

How to Make Reactions Go Faster! Ø Catalysts Ø Temperature Ø Lowers the activation energy of a reaction Ø Is not consumed during the reaction Ø Ø Increases kinetic energy and causes more collisions Ø Increases number of collisions Agitation
How to Make Reactions Go Faster! Ø Surface Area Ø Causes more molecules to be exposed Ø Increases number of collisions Ø Concentration Ø Ø Increased number of particles Increased number of collisions
- Slides: 19