Unit 6 Atomic Theory Day 4 Bohr Model

Unit 6: Atomic Theory Day 4: Bohr Model

Warm Up OPEN NOTEBOOK: Page 3 WRITE ON TOP (in big letters): Unit 6 Warm Ups THEN: Turn to Periodic Table FIND: Plutonium ANSWER: The following questions in Warm Up Section How many protons does Plutonium have? Neutrons? Electrons? TIME: 4 MINUTES WHEN DONE: Tape “Atoms are Building Blocks” Reading (from last class) to page 7 and “Atomic Structure Practice” to page 8 (look for handed back work in blue bin)
Agenda Bohr Model Flame Test Demonstration Bohr Model Practice
Learning Targets 3 - I can identify the number of protons, neutrons and electrons in an atom of any element 4 - I can use the periodic table to create Bohr models and Lewis Dot Structures for any of the first 20 elements.

Bohr Model Notes OPEN: Notebook and open to pages 9 and 10 GLUE IN: Bohr Model Notes (in blue bin) TIME: 1 MINUTE WHEN DONE: Find your favorite number and match it to the element on Periodic Table
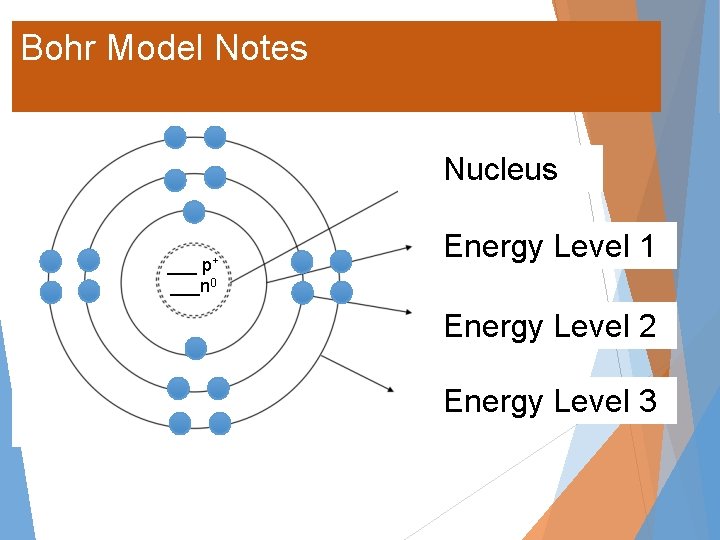
Bohr Model Notes Nucleus ___ p+ ___n 0 Energy Level 1 Energy Level 2 Energy Level 3
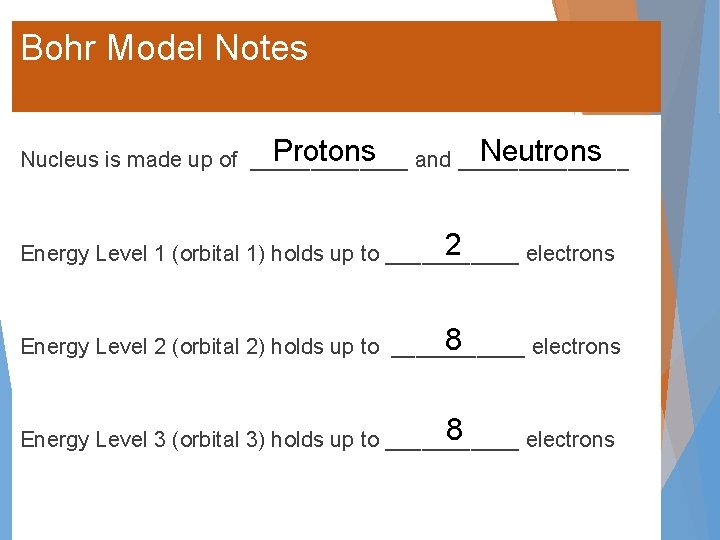
Bohr Model Notes Protons and _______ Neutrons Nucleus is made up of _______ 2 Energy Level 1 (orbital 1) holds up to ______ electrons 8 Energy Level 2 (orbital 2) holds up to ______ electrons 8 Energy Level 3 (orbital 3) holds up to ______ electrons
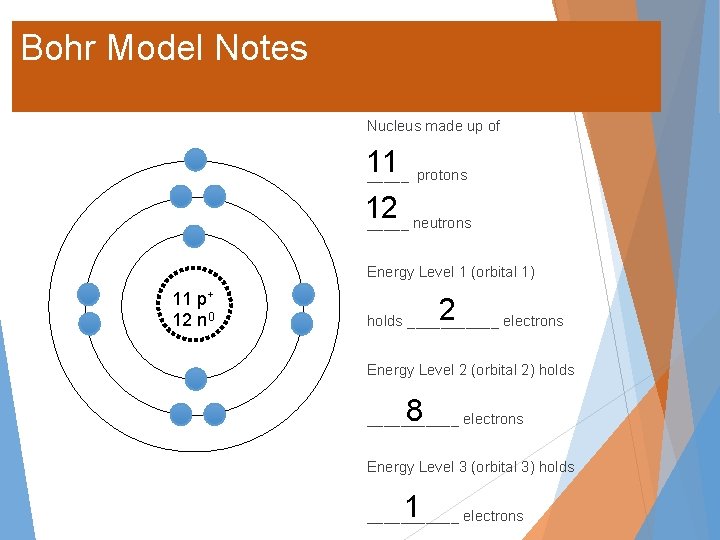
Bohr Model Notes Nucleus made up of 11 _____ protons 12 _____ neutrons Energy Level 1 (orbital 1) 11 p+ 12 n 0 2 holds ______ electrons Energy Level 2 (orbital 2) holds 8 ______ electrons Energy Level 3 (orbital 3) holds 1 ______ electrons

Flame Test Demo As you watch the demonstration, try to answer this question: What part of an atom creates different colors in fireworks?

Flame Test Demo Why is Lithium pinkish purple? Why is Copper green?
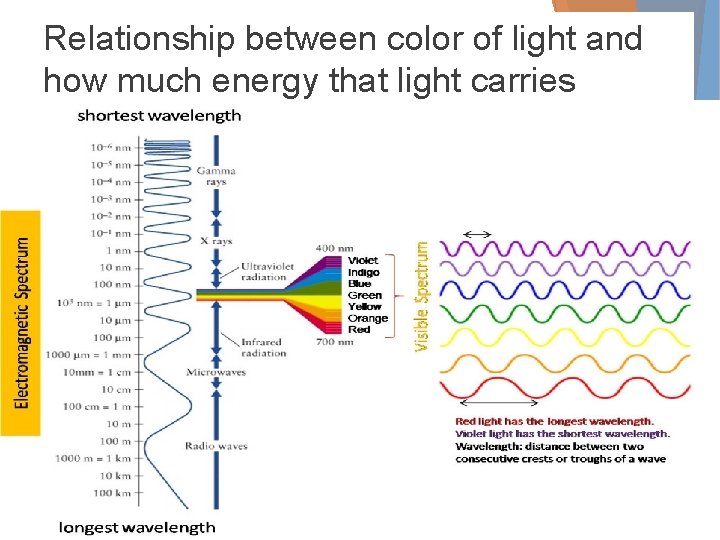
Relationship between color of light and how much energy that light carries
Flame Test Demo The electrons are “energized” by the flame When these electrons are “energized” they jump to a higher energy level As they come back to their original energy level, they release different colors!!! The color depends on how much energy was absorbed and then released
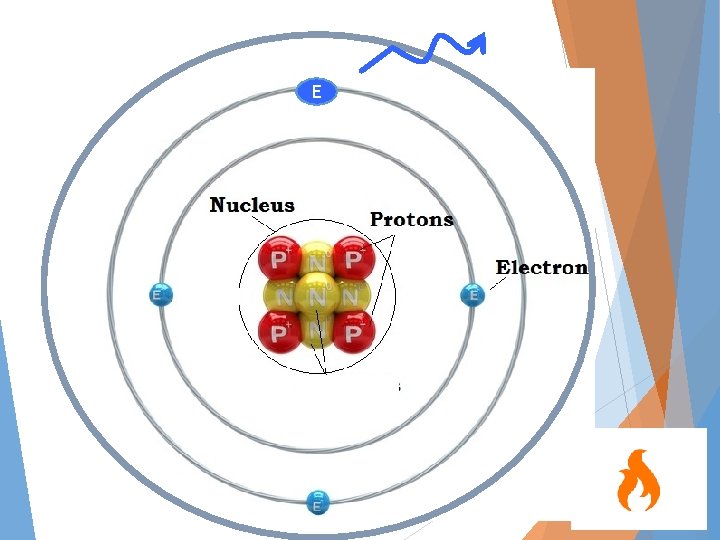
E

Bohr Model Practice COMPLETE: First two elements of Bohr Model Practice USE: Bohr Model Notes (Page 9 and 10 in your notebook) Calculator Table Partners REMEMBER: Steps to calculating # of neutrons THEN: Complete the remaining for 4 (I will assign you) TIME: 22 MINUTES WHEN DONE: Turn into class box and sign up for the element you want to build and obtain the Bohr Model Rough Draft
- Slides: 14