Unit 6 Aqueous Reactions and Solution Stoichiometry Solutions





- Slides: 17

Unit 6 – Aqueous Reactions and Solution Stoichiometry

Solutions and Electrolytes Solution is a homogeneous mixture of two or more substances Solvent Solute Electrolyte form ions in aqueous solution Ionic compounds H 2 O Non electrolyte do not form ions in solution Molecular compounds

Strong vs Weak Electrolytes Electrolyte strength determined amount of ions present in aqueous solutions Strong Electrolytes – dissolved compound that exists mainly or completely as ions Acids, Ionic Compounds HCl → H+ + Cl- Weak Electrolytes – dissolved compound that exists mainly as molecules not ions HC 2 H 3 O 2 ↔ H+ + C 2 H 3 O 2 -

Precipitation Reactions Precipitation reactions result in formation of insoluble solid Solubility is the amount of a substance that can be dissolved in a large amount of solvent Compound with solubility less than 0. 01 mol/L is insoluble Can not determine solubility based on physical properties

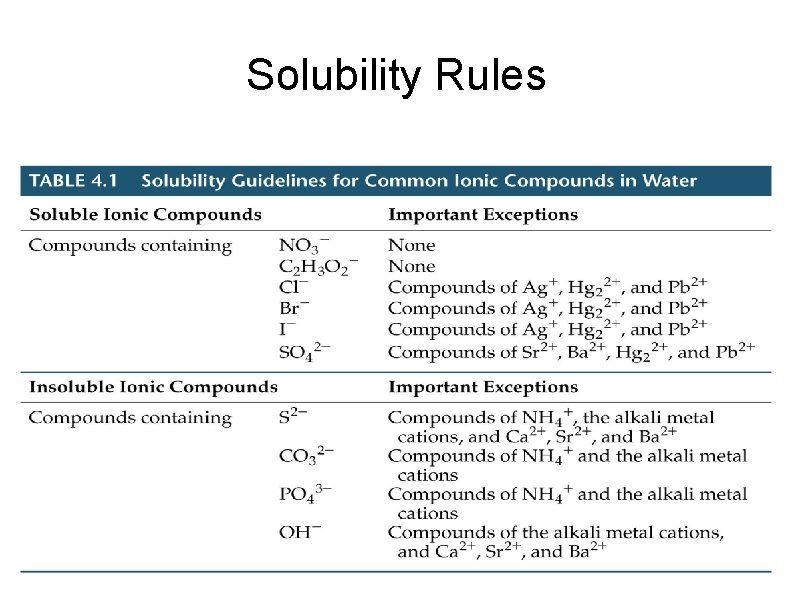
Solubility Rules

Predicting Solubility Determine whether the following compounds are soluble or insoluble and why. Sodium carbonate Lead sulfate Barium nitrate Cobalt (II) hydroxide Ammonium phosphate

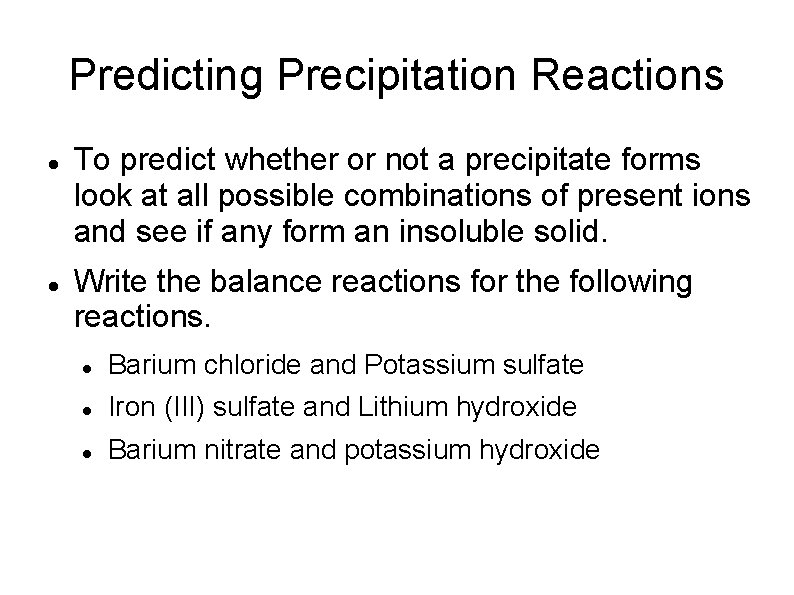
Predicting Precipitation Reactions To predict whether or not a precipitate forms look at all possible combinations of present ions and see if any form an insoluble solid. Write the balance reactions for the following reactions. Barium chloride and Potassium sulfate Iron (III) sulfate and Lithium hydroxide Barium nitrate and potassium hydroxide

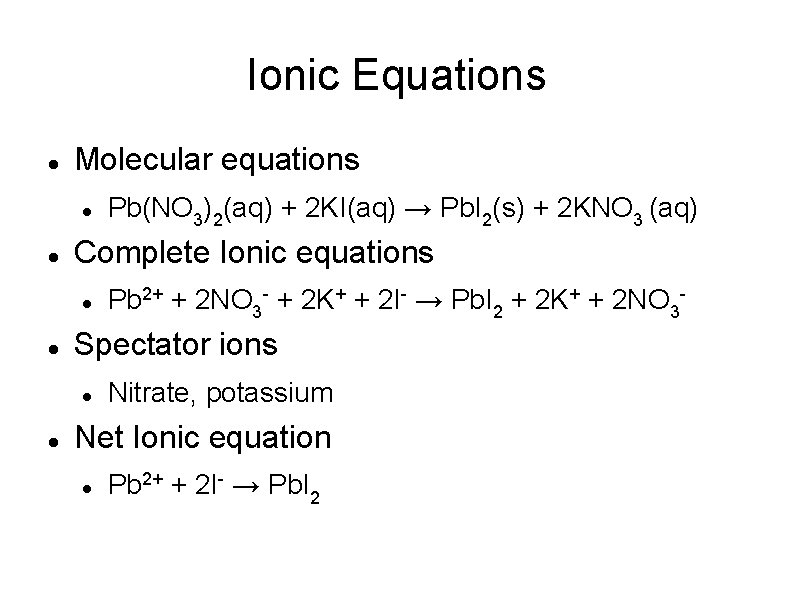
Ionic Equations Molecular equations Complete Ionic equations Pb 2+ + 2 NO 3 - + 2 K+ + 2 I- → Pb. I 2 + 2 K+ + 2 NO 3 - Spectator ions Pb(NO 3)2(aq) + 2 KI(aq) → Pb. I 2(s) + 2 KNO 3 (aq) Nitrate, potassium Net Ionic equation Pb 2+ + 2 I- → Pb. I 2

Writing Net Ionic Equations 1. Write balanced reactions 2. Rewrite equation to show ions in solution 3. Identify and cancel spectator ions If all ions are spectator ions no reaction occurs

Net Ionic Equation Practice Write the net ionic equations for the reactions between: Calcium chloride and sodium carbonate Silver nitrate and potassium phosphate

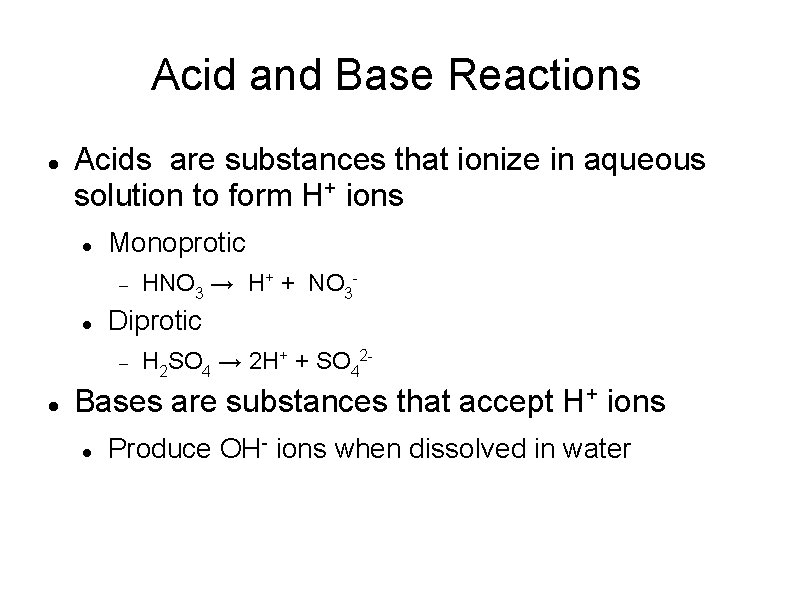
Acid and Base Reactions Acids are substances that ionize in aqueous solution to form H+ ions Monoprotic Diprotic HNO 3 → H+ + NO 3 H 2 SO 4 → 2 H+ + SO 42 - Bases are substances that accept H+ ions Produce OH- ions when dissolved in water

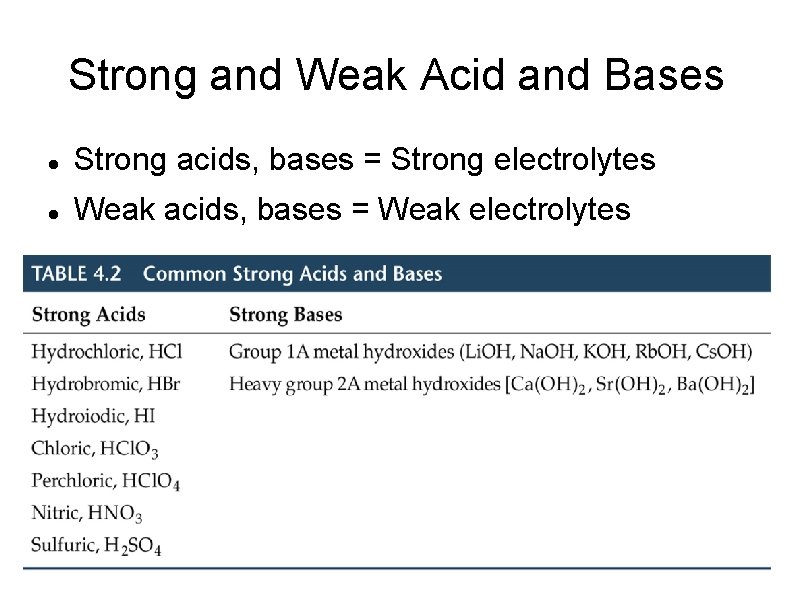
Strong and Weak Acid and Bases Strong acids, bases = Strong electrolytes Weak acids, bases = Weak electrolytes

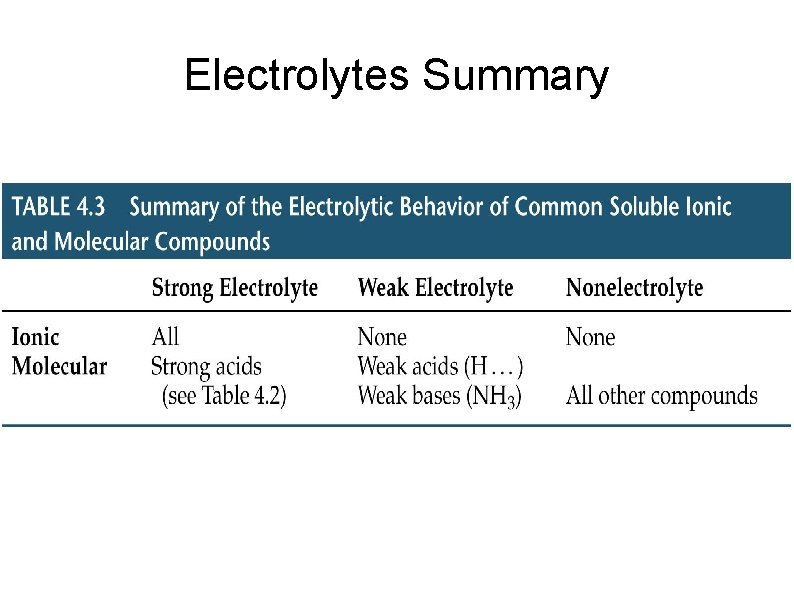
Electrolytes Summary

Classifying Electrolytes Classify the following substances as strong, weak or nonelectrolytes. Calcium chloride Nitric Acid Ethanol (C 2 H 5 OH) Formic Acid (HCHO 2) Potassium hydroxide Rank solutions of calcium nitrate, table sugar, sodium acetate, and acetic acid in order of increasing conductivity.

Neutralization Reactions Acids and base can change the color of dyes Litmus Mix acids and bases and neutralization reaction occurs HCl + Na. OH → H 2 O + Na. Cl H+ + OH- → H 2 O Write the net ionic equation for reactions between Hydrochloric acid and magnesium hydroxide Acetic acid and barium hydroxide

Acid base Reactions that form gases Bases other than OH- may react with acids to form molecules 2 HCl + Na 2 S → H 2 S + 2 Na. Cl 2 H+ + S 2 - → H 2 S Carbonates and bicarbonates react with acids to form CO 2 HCl + Na. HCO 3 → Na. Cl + H 2 CO 3 → H 2 O + CO 2 HCl + Na. HCO 3 → Na. Cl + H 2 O + CO 2 H+ + HCO 3 - → H 2 O + CO 2

Homework 3, 5, 10, 11, 14, 15, 18, 23, 26, 30, 31