Unit 5 The Periodic Table Chemical Bonding Objective

Unit 5 – The Periodic Table & Chemical Bonding

Objective 1 �Identify the position of groups, periods, and different chemical families on the periodic table.
Periodic Table �Dmitri Mendeleev – mid 1800’s �Proposed a table for 70 elements based on mass and properties �Henry Moseley – 1913 �Determined the atomic number of elements and arranged the table in order of atomic number
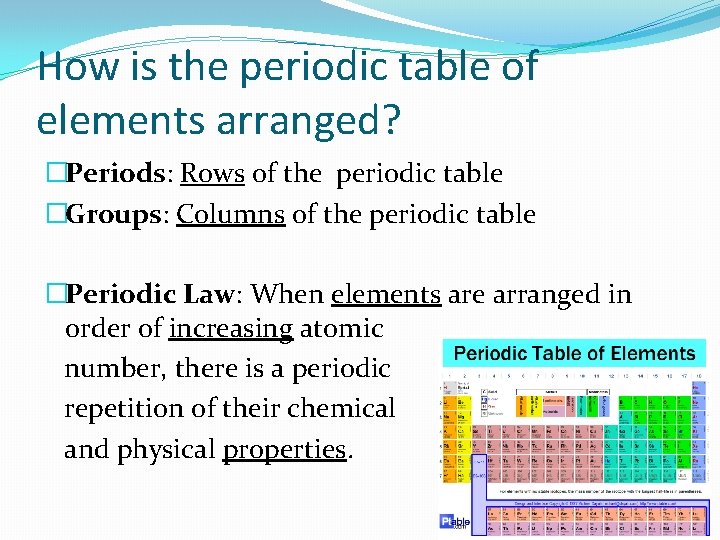
How is the periodic table of elements arranged? �Periods: Rows of the periodic table �Groups: Columns of the periodic table �Periodic Law: When elements are arranged in order of increasing atomic number, there is a periodic repetition of their chemical and physical properties.

Groupings to know on the Periodic Table (Fill in on your notes. ) �Elements (H, He) �Metals �Non-metals �Metalloids �Transition Metals �Lanthinides �Actinides �Alkali Metals �Alkaline Earth Metals �Halogens �Noble (inert) gases

Properties of Metals �Metals: �Bright metallic luster �Solids are easily deformed �Good conductors of electricity and heat

Properties of Nonmetals �Nonmetals: �Non-lustrous, various colors �Solids may be hard or soft, usually brittle �Poor conductors of electricity
Properties of Metalloids �Metalloids have properties intermediate between metals and nonmetals.

Objective 2 �Identify forces between atoms.
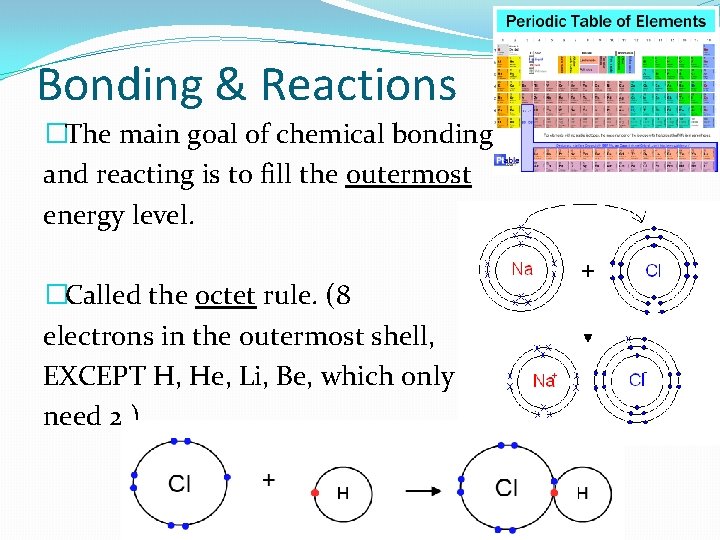
Bonding & Reactions �The main goal of chemical bonding and reacting is to fill the outermost energy level. �Called the octet rule. (8 electrons in the outermost shell, EXCEPT H, He, Li, Be, which only need 2. )
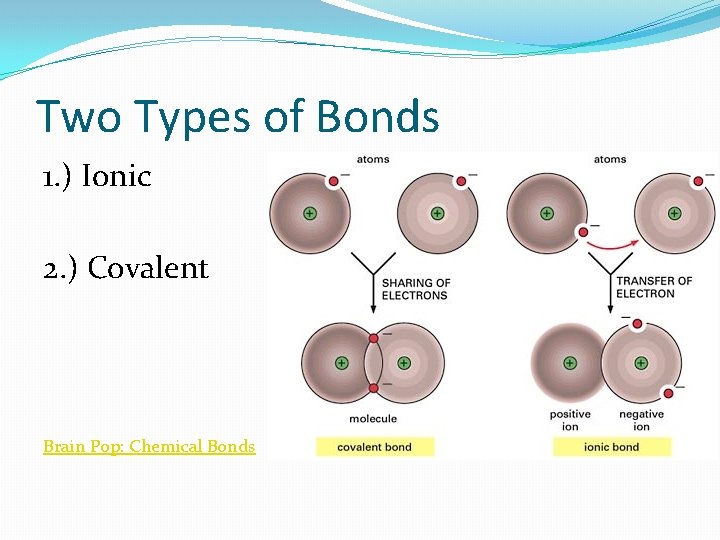
Two Types of Bonds 1. ) Ionic 2. ) Covalent Brain Pop: Chemical Bonds
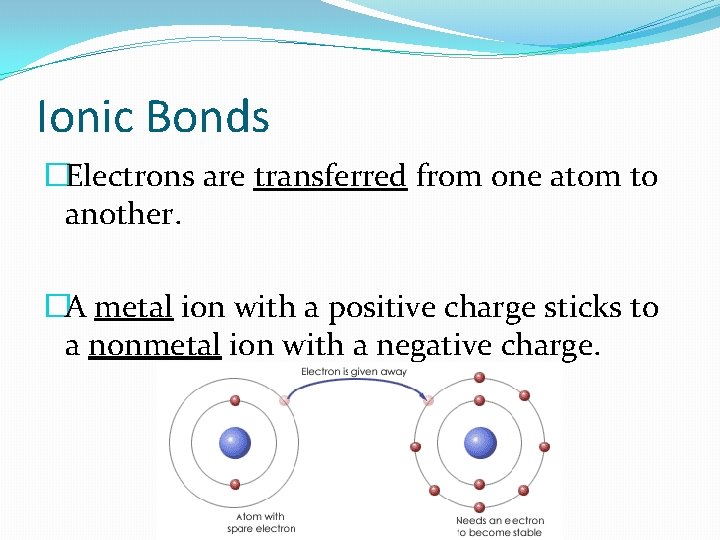
Ionic Bonds �Electrons are transferred from one atom to another. �A metal ion with a positive charge sticks to a nonmetal ion with a negative charge.
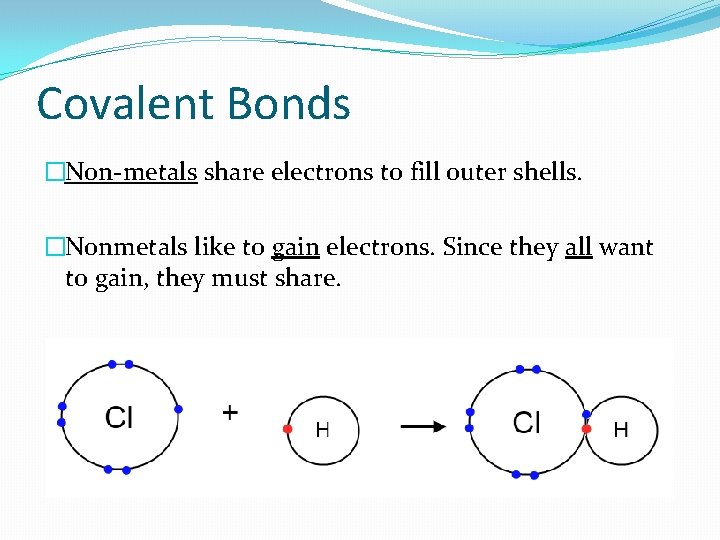
Covalent Bonds �Non-metals share electrons to fill outer shells. �Nonmetals like to gain electrons. Since they all want to gain, they must share.
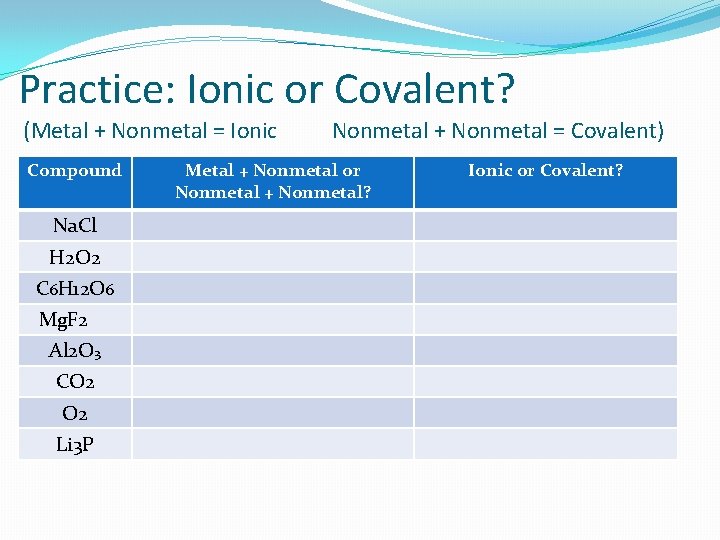
Practice: Ionic or Covalent? (Metal + Nonmetal = Ionic Compound Na. Cl H 2 O 2 C 6 H 12 O 6 Mg. F 2 Al 2 O 3 CO 2 Li 3 P Nonmetal + Nonmetal = Covalent) Metal + Nonmetal or Nonmetal + Nonmetal? Ionic or Covalent?

Objective 3 �Diagram compounds with Lewis dot structures.
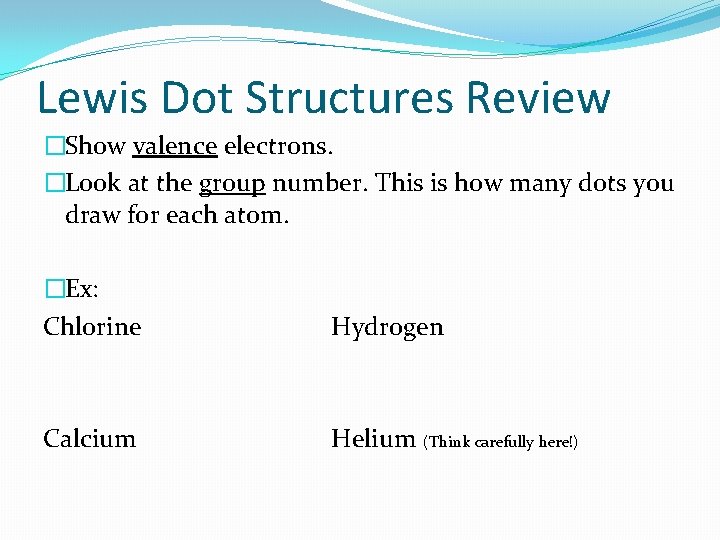
Lewis Dot Structures Review �Show valence electrons. �Look at the group number. This is how many dots you draw for each atom. �Ex: Chlorine Hydrogen Calcium Helium (Think carefully here!)
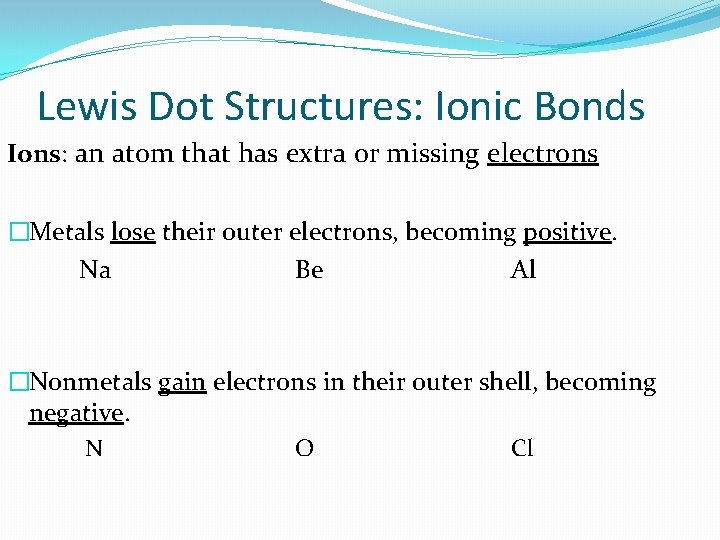
Lewis Dot Structures: Ionic Bonds Ions: an atom that has extra or missing electrons �Metals lose their outer electrons, becoming positive. Na Be Al �Nonmetals gain electrons in their outer shell, becoming negative. N O Cl

Where do these gained or lost electrons go? �Ex: Sodium chloride Metal = sodium �Ex: Magnesium oxide Metal = magnesium nonmetal = chlorine nonmetal = oxygen

Use the valence electrons! �To find the charge of an ion, look at the valence electrons and count how many they need to give away or add to reach a full energy level (8 electrons for most. 2 electrons for H, He, Li, Be) �Ex: What charge will the following ions have? sodium magnesium phosphorous carbon bromine sulfur
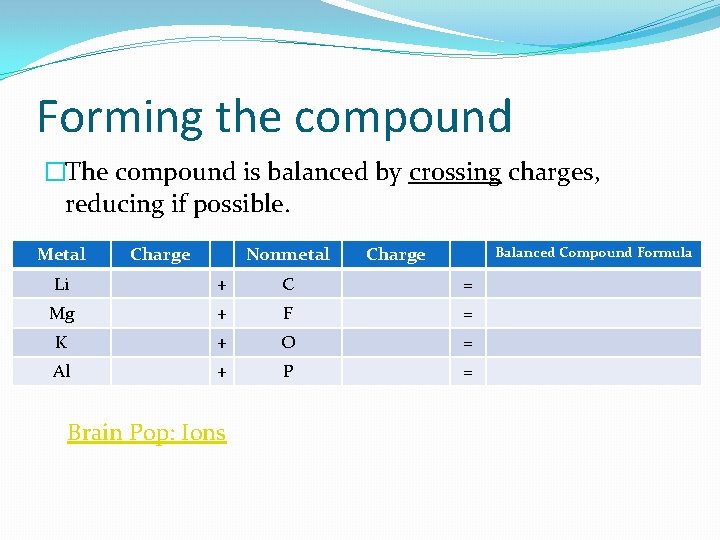
Forming the compound �The compound is balanced by crossing charges, reducing if possible. Metal Charge Nonmetal Charge Balanced Compound Formula Li + C = Mg + F = K + O = Al + P = Brain Pop: Ions
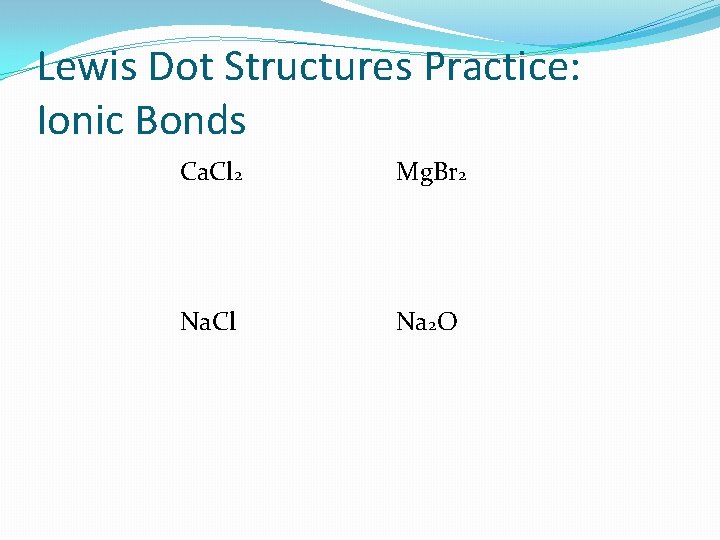
Lewis Dot Structures Practice: Ionic Bonds Ca. Cl 2 Mg. Br 2 Na. Cl Na 2 O
Lewis Dot Structures: Covalent Bonds �Non-metals share electrons to fill outer shells. � 1 bond = 2 electrons, 2 bonds = 4 electrons, 3 bonds = 6 electrons �Follow these rules: (Ex: NCl 3) 1. ) Look at the formula. Then add up total valence electrons needed. 2. ) Single bond all of the atoms, picking a center atom when possible. (H is never in the middle. ) 3. ) Fill every atoms energy level. Remember, most need 8 electrons, but H only needs 2. 4. ) Count the electrons & erase some if you have too many. Move the electrons into double or triple bonds if needed.
Lewis Dot Structures Practice: Covalent Bonds N 2 Cl 2 O 2 CI 4 CBr 4
- Slides: 23