Unit 5 Pure Substances Mixtures Classifying Matter Pure

Unit 5 – Pure Substances & Mixtures
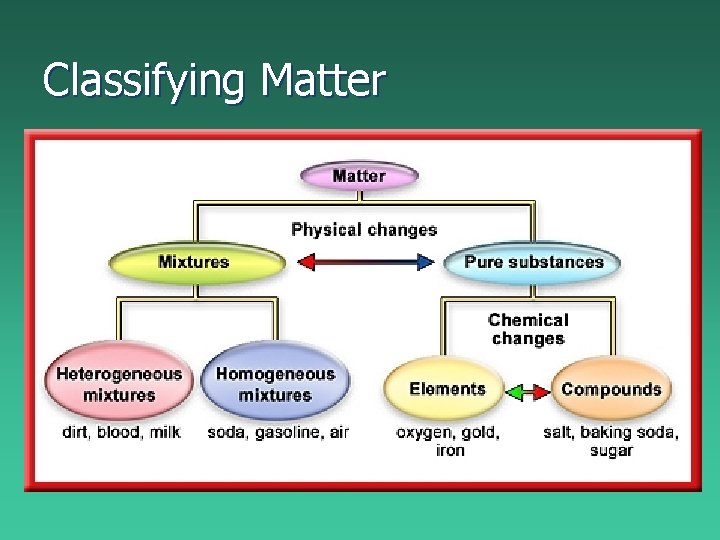
Classifying Matter

Pure Substances • Pure substances cannot be separated by physical means – Elements: cannot be chemically separated, listed on the periodic table carbon (C) sulfur (S) copper (Cu) mercury (Hg) – Compounds: can be chemically separated, made up of elements salt (Na. Cl) water (H 2 O) sugar (C 6 H 12 O 6) rust (Fe 2 O 3)
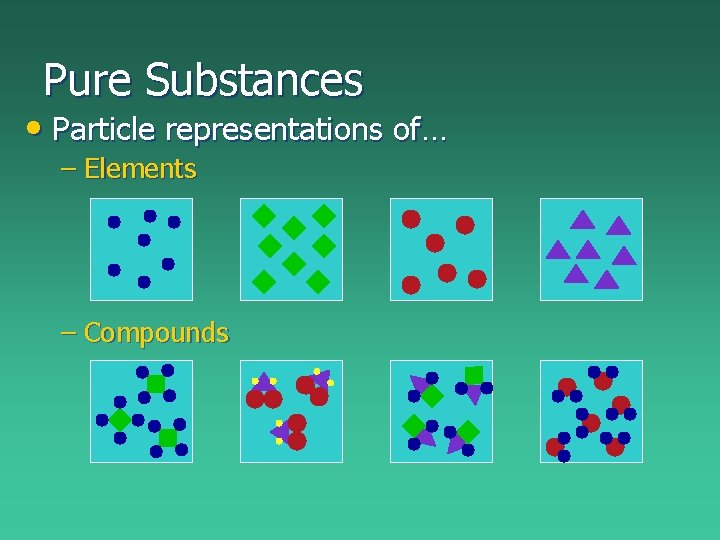
Pure Substances • Particle representations of… – Elements – Compounds
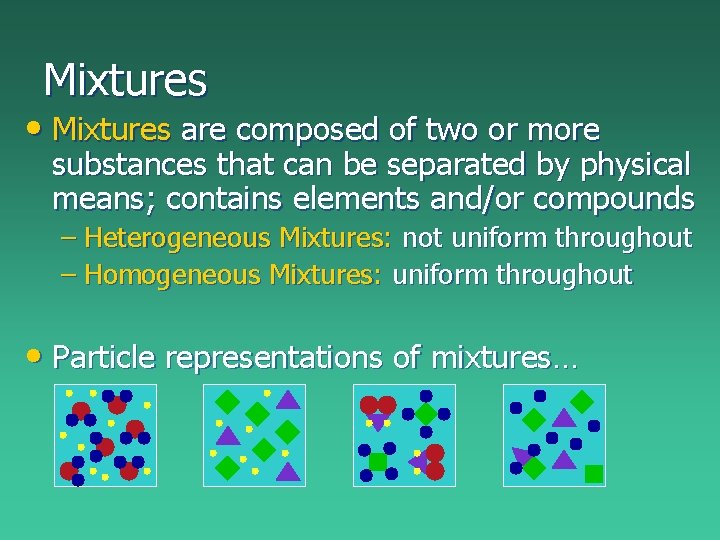
Mixtures • Mixtures are composed of two or more substances that can be separated by physical means; contains elements and/or compounds – Heterogeneous Mixtures: not uniform throughout – Homogeneous Mixtures: uniform throughout • Particle representations of mixtures…

Heterogeneous Mixtures • Heterogeneous Mixture: two or more substances physically combined; not uniform throughout • Ex: Granite, chex mix

Homogeneous Mixtures • Homogeneous Mixture: two or more substances • physically combined; uniform throughout Known as a solution composed of… – Solute: substance being dissolved (smaller amount) – Solvent: substance that does the dissolving (larger amount) • Examples: milk, kool-aid, brass

Solutions - Rate • Rate of Solvation (Dissolving) – how fast a solute will dissolve in a solvent • Increase rate of solution by: – Heating – Stirring – Crushing

Solutions - Solubility • Soluble: able to be dissolved – Sugar is soluble in water • Insoluble: unable to be dissolved – Oil is insoluble in water • Solubility: the amount of solute that will dissolve in a solvent – Depends on temperature and pressure

Solutions - Solubility • Factors that affect solubility – Temperature • Solids: ↑ temp ↑ solubility • Gases: ↑ temp ↓ solubilty – Ex: Coffee, thermal pollution – Pressure • Solids: no effect • Gases: ↑ pressure ↑ solubility – Ex: Carbonated drinks

Solutions - Types • Unsaturated – solvent contains less solute than it can hold • Saturated – solvent contains the maximum amount of solute – If more solute is added, it does not dissolve Ex: Coffee and sugar How could you tell if your coffee was unsaturated or saturated?

Solutions - Types • Supersaturated – contains more solute than the solvent can normally hold (ex: sodium acetate) – Made by heating the solution to dissolve the excess, then cooling to a lower temperature
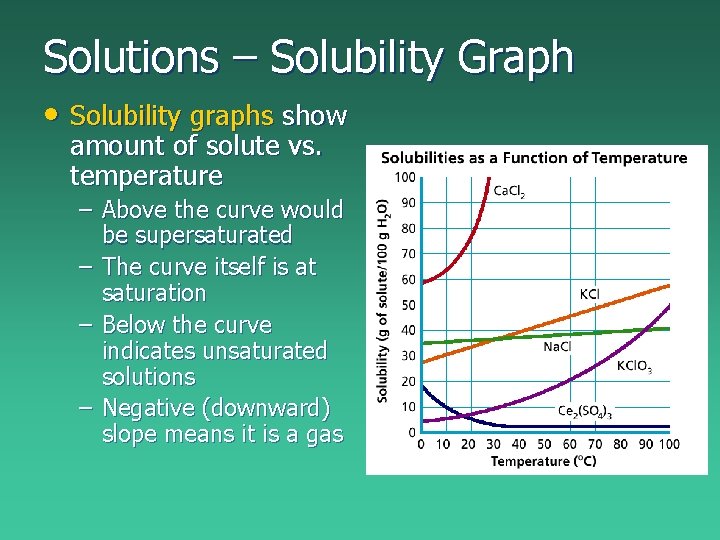
Solutions – Solubility Graph • Solubility graphs show amount of solute vs. temperature – Above the curve would be supersaturated – The curve itself is at saturation – Below the curve indicates unsaturated solutions – Negative (downward) slope means it is a gas

Solutions - Colligative Properties • Colligative properties (physical property) depend on the concentration of the particles in the solution – Examples: • Boiling point elevation: adding salt to water for cooking • Freezing point depression: salting the roads before a freeze, antifreeze in cars, and making homemade ice cream • Osmotic pressure: responsible for plant’s cell wall, sturdiness

Solutions - Colligative Properties • How does adding a solute change physical properties? – Solute particles get in the way of the solvent molecules – Makes it harder for the solvent molecules to boil (more energy needed – higher temperature) – Makes it harder for the solvent molecules to freeze (need to release more energy – lower temperature)

Solutions - Concentration • Concentration is the amount of solute dissolved in • a given amount of solvent Described qualitatively as… – Dilute – solution containing a small amount of solute – Concentrated – solution containing a large amount of solute
Solutions - Molarity • Molarity (M) describes concentration quantitatively – The number of moles of solute dissolved in 1 liter of a solution – Ex: molarity of IV fluids is calculated before it is administered to the patient – Equation: Molarity = moles of solute liters of solution also written as… M = mol L
Solutions - Molarity 1. What is the molarity of 2 mol sodium chloride in 5 L of solution? 0. 4 M 2. How many moles of potassium bromide would be present in 1 L of a 3 M solution? 3 moles 3. What is the volume of a 1. 5 M solution of hydrochloric acid that contains 10. 0 moles? 6. 7 L

Solutions – Dilutions M 1 V 1 = M 2 V 2 • How many liters of a 3. 00 M KI stock solution would you use to make 0. 300 L of a 1. 25 M KI solution?

Solutions – Dilutions M 1 V 1 = M 2 V 2 • How many milliliters of a 5. 0 M H 2 SO 4 stock solution would you need to prepare 100. 0 m. L of 0. 25 M H 2 SO 4?

Solutions – Dilutions M 1 V 1 = M 2 V 2 • How many milliliters of a solution of 4. 00 M KI are needed to prepare 250. 0 m. L of 0. 760 M KI?

Solutions – Dilutions M 1 V 1 = M 2 V 2 • How could you prepare 250 m. L of 0. 20 M Na. Cl using only a solution of 1. 0 M Na. Cl and water?
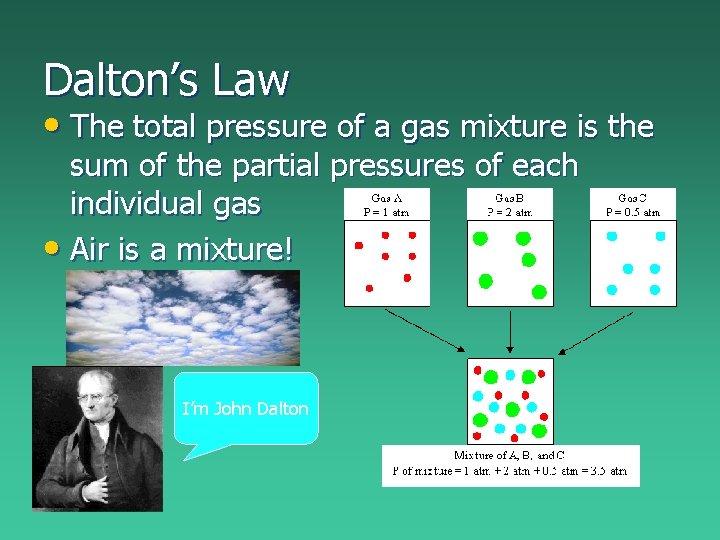
Dalton’s Law • The total pressure of a gas mixture is the sum of the partial pressures of each individual gas • Air is a mixture! I’m John Dalton

Dalton’s Law • Ex: The pressure on a tank of air with… + 20. 9 atm oxygen Ptotal + 78. 1 atm nitrogen + 0. 97 atm argon + 1. 28 atm water vapor + 0. 05 atm carbon dioxide = 101. 3 atm = P 1 + P 2 + P 3…

Dalton’s Law • Determine the total pressure of a gas mixture that contains oxygen, nitrogen, and helium. The partial pressures are: PO 2 = 20. 0 k. Pa, PN 2 = 46. 7 k. Pa, and PHe = 26. 7 k. Pa.

Dalton’s Law Air contains oxygen, nitrogen, carbon dioxide, and other gases. What is the partial pressure of oxygen (PO 2) at 101. 3 k. Pa of total pressure if the partial pressures of nitrogen, carbon dioxide, and other gases are 79. 10 k. Pa, 0. 040 k. Pa, and 0. 94 k. Pa, respectively?
Separating a Mixture • Separating a mixture - components are separated without changing their physical identity – Manual Separation – Magnetism – Filtration – Evaporation – Distillation – Centrifuging – Chromatography

Manual Separation • Decanting – Separates two liquids of different densities by pouring • Sifting – Separates two solids of different particle size • Sorting – Separates two solids by picking

Magnetism • Separates metals (such as iron) from a mixture

Filtration • Separates solid substances from liquids and solutions

Evaporation • Separates a dissolved solid from its solvent

Distillation • Separates homogeneous mixture with different boiling points (heat mixture and catch condensed vapor)
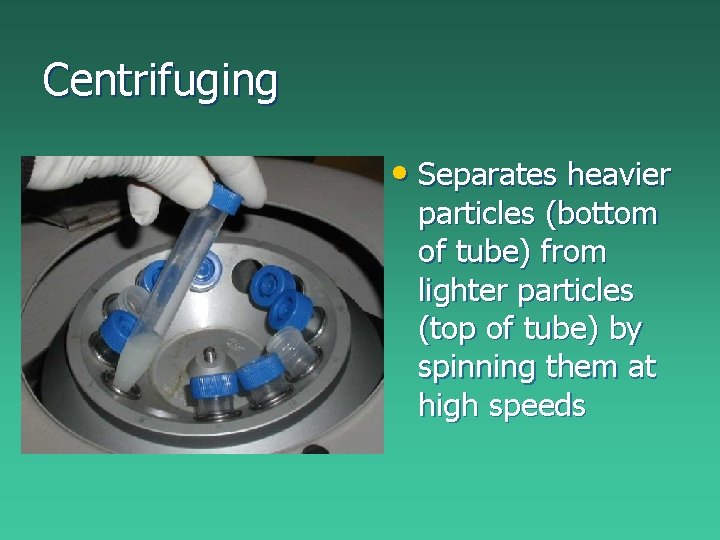
Centrifuging • Separates heavier particles (bottom of tube) from lighter particles (top of tube) by spinning them at high speeds
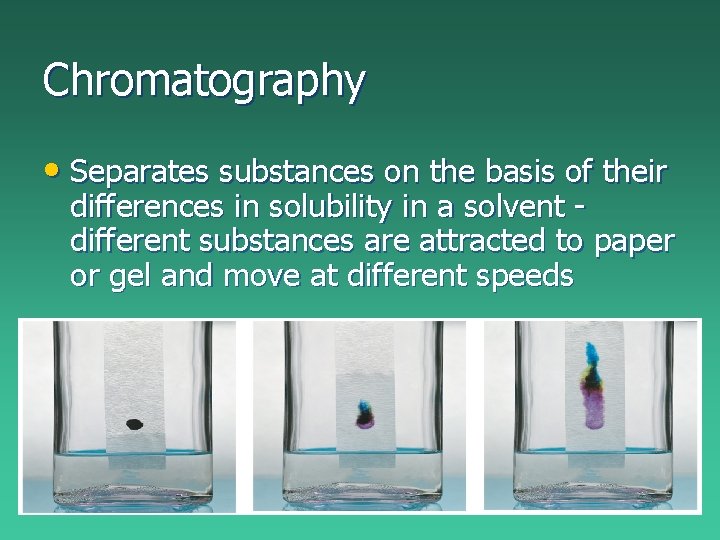
Chromatography • Separates substances on the basis of their differences in solubility in a solvent different substances are attracted to paper or gel and move at different speeds
- Slides: 34