Unit 5 Naming Acids and Bases Acids Acid

Unit 5 Naming Acids and Bases
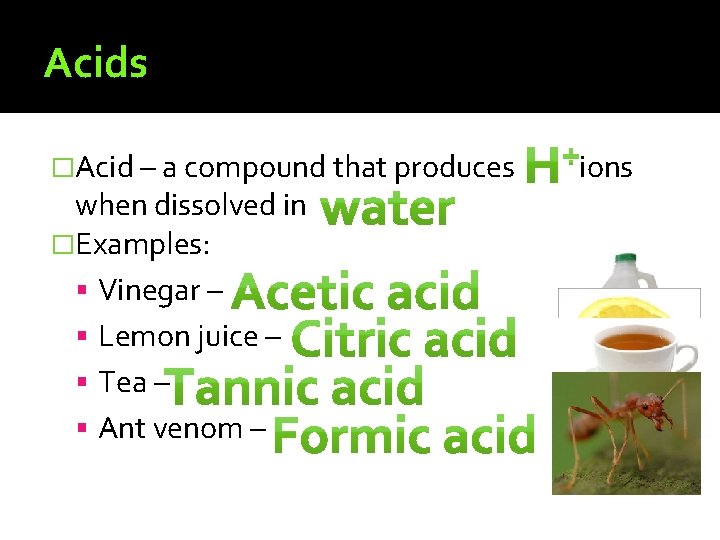
Acids �Acid – a compound that produces when dissolved in �Examples: Vinegar – Lemon juice – Tea – Ant venom – ions

Properties of Acids � taste � �Turns litmus paper �Reacts with metals to form gas � solutions of acids are water!) �Reacts with (must be mixed with to form and

Properties of Acids �Sugar, corn syrup, modified corn starch, citric acid, tartaric acid, natural and artificial flavors, yellow 5, yellow 6, red 40, blue 1 �What ingredients make these… so sour?

Naming Binary Acids �H + one other element �Begin with �Use the of the element name �Add the suffix �HCl “hydro-” root “-ic” hydro ic acid
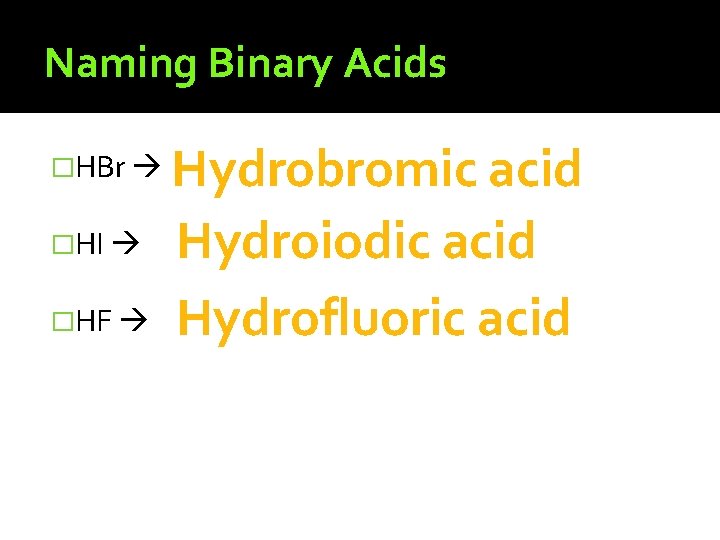
Naming Binary Acids �HBr �HI �HF Hydrobromic acid Hydroiodic acid Hydrofluoric acid

Naming Ternary Acids �H + polyatomic ion �Begin with the �Add suffix �HNO 3 ion without if there was an

Naming Ternary Acids �HCl. O 3 �H 3 PO 3 �H 2 CO 3

Bases �Base – a compound that produces �Examples: when dissolved in ions Milk of Magnesia – neutralizes stomach acid Drain cleaner–
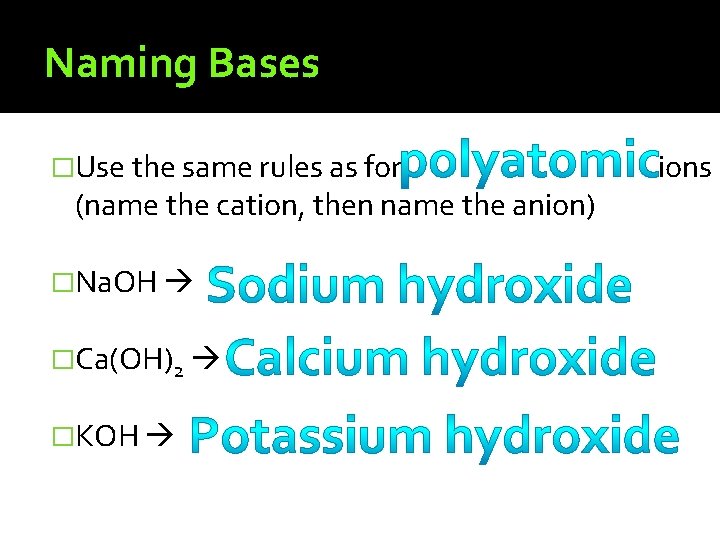
Naming Bases �Use the same rules as for (name the cation, then name the anion) �Na. OH �Ca(OH)2 �KOH ions
- Slides: 10