UNIT 5 LESSON 2 Synthesis Combustion and Decomposition

UNIT 5 LESSON 2 Synthesis, Combustion, and Decomposition Reactions 1

Types of Reactions 1. 2. 3. 4. 5. Video Link Synthesis Reaction Decomposition Reaction Single-Replacement Reaction Double-Replacement Reaction Combustion Reaction More is listed about each type on page 6 of your reference packet 2
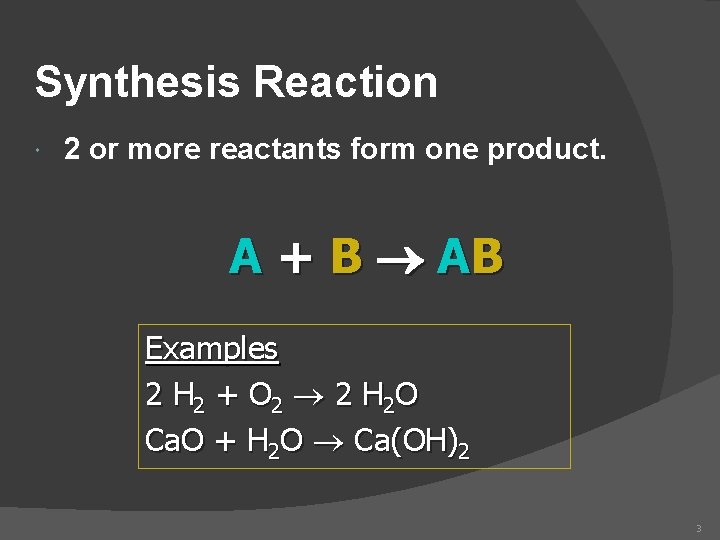
Synthesis Reaction 2 or more reactants form one product. A + B AB Examples 2 H 2 + O 2 2 H 2 O Ca. O + H 2 O Ca(OH)2 3

Combustion Reaction ► An element or compound (generally a hydrocarbon) reacts with oxygen producing carbon dioxide, water, and heat. ► This is a special type of synthesis Example C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O 4

Decomposition Reaction One reactant forms 2 or more products. AB A + B Examples 2 Hg. O 2 Hg + O 2 2 Na. Cl 2 Na + Cl 2 5

• Decomposition • Binary: split apart into two elements. • Ag₂O Ag + O 2 • If not binary, refer to reference packet page 6 • “M” means any metal • Example – Metal Carbonate • Na 2 CO 3 Na 2 O + CO 2 • Example – Metal hydroxide • Ca(OH)2 Ca. O + H 2 O 6
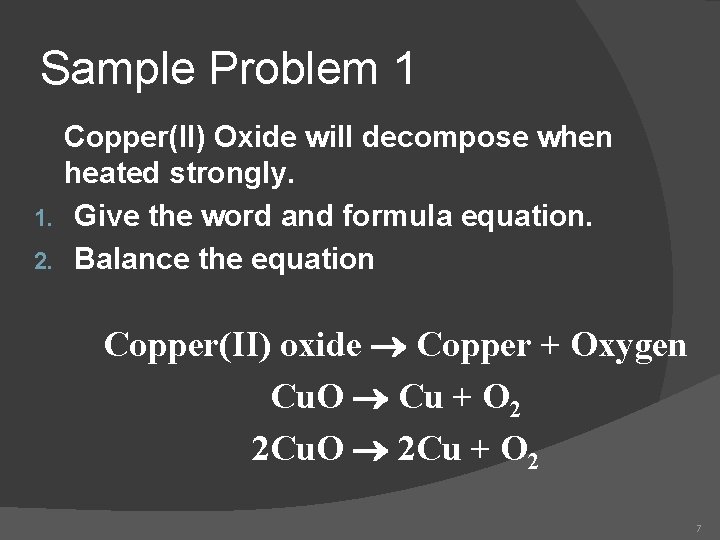
Sample Problem 1 Copper(II) Oxide will decompose when heated strongly. 1. Give the word and formula equation. 2. Balance the equation Copper(II) oxide Copper + Oxygen Cu. O Cu + O 2 2 Cu. O 2 Cu + O 2 7
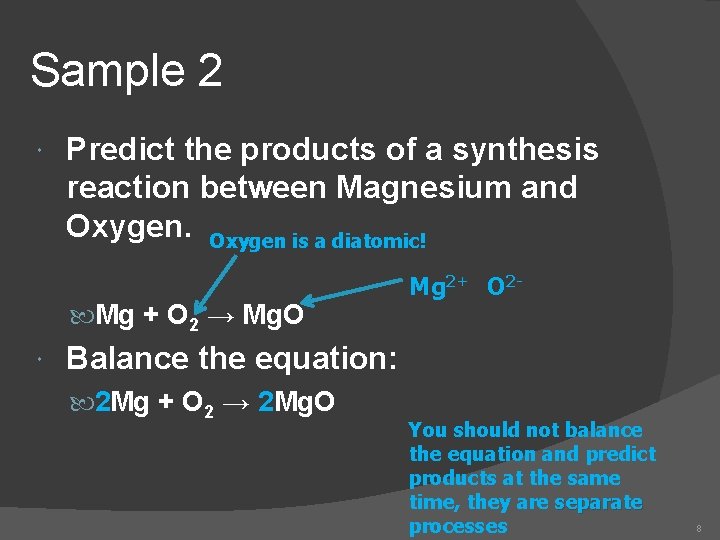
Sample 2 Predict the products of a synthesis reaction between Magnesium and Oxygen is a diatomic! Mg + O 2 → Mg. O Mg 2+ O 2 - Balance the equation: 2 Mg + O 2 → 2 Mg. O You should not balance the equation and predict products at the same time, they are separate processes 8

Sample 3 What products will be formed when Al 2(CO 3)3 decomposes? Balance the equation AFTER predicting the products Al 2(CO 3)3 →? ? Look at reference packet Al 2(CO 3)3 → Al 2 O 3 + CO 2 ___Al 2(CO 3)3 → ___Al 2 O 3 + ___CO 2 Al 2(CO 3)3 → Al 2 O 3 + 3 CO 2 9
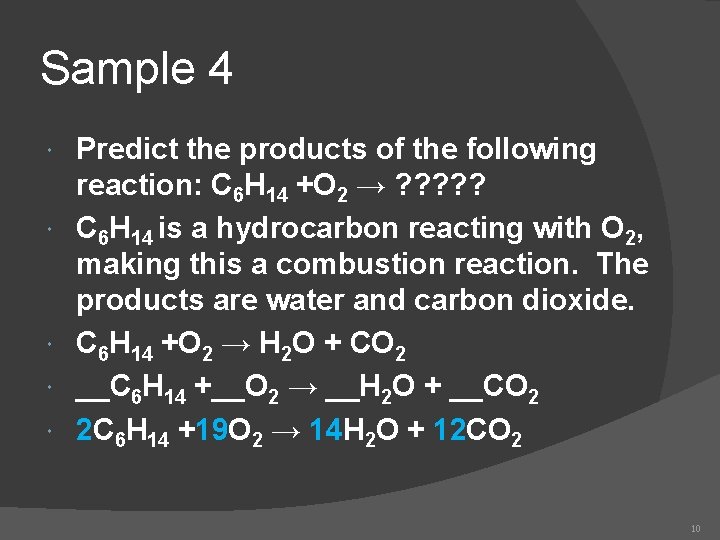
Sample 4 Predict the products of the following reaction: C 6 H 14 +O 2 → ? ? ? C 6 H 14 is a hydrocarbon reacting with O 2, making this a combustion reaction. The products are water and carbon dioxide. C 6 H 14 +O 2 → H 2 O + CO 2 __C 6 H 14 +__O 2 → __H 2 O + __CO 2 2 C 6 H 14 +19 O 2 → 14 H 2 O + 12 CO 2 10
- Slides: 10