Unit 5 Ionic Compounds Chemical Bonds Three students
Unit 5: Ionic Compounds
Chemical Bonds: Three students were discussing their ideas about chemical bonds. This is what they said: � Jane: “I think a chemical bond is produced by a molecule. It is a substance made up of matter that holds atoms together. ” � Will: “I think a chemical bond is an attraction between atoms. It is not made up of matter. ” � Leta: “I think a chemical bond is a structural part of an atom that connects it to other atoms. ” � What is a chemical bond? � Do you know any types of bonds?
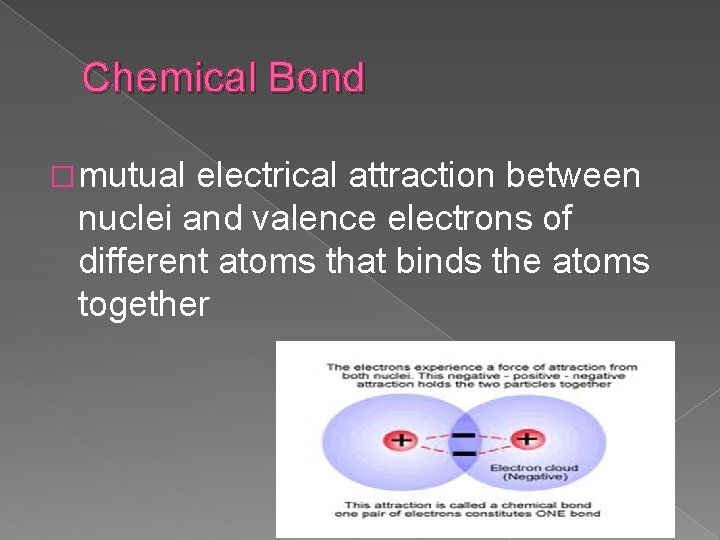
Chemical Bond � mutual electrical attraction between nuclei and valence electrons of different atoms that binds the atoms together
Chemical Formulas � Shows the kinds and numbers of atoms in the smallest representative unit of a substance � Symbols show the type of atom � Subscripts show many atoms
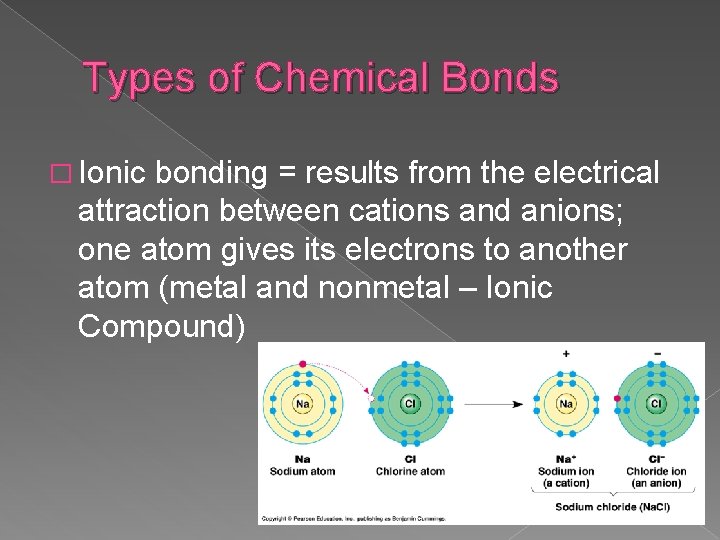
Types of Chemical Bonds � Ionic bonding = results from the electrical attraction between cations and anions; one atom gives its electrons to another atom (metal and nonmetal – Ionic Compound)

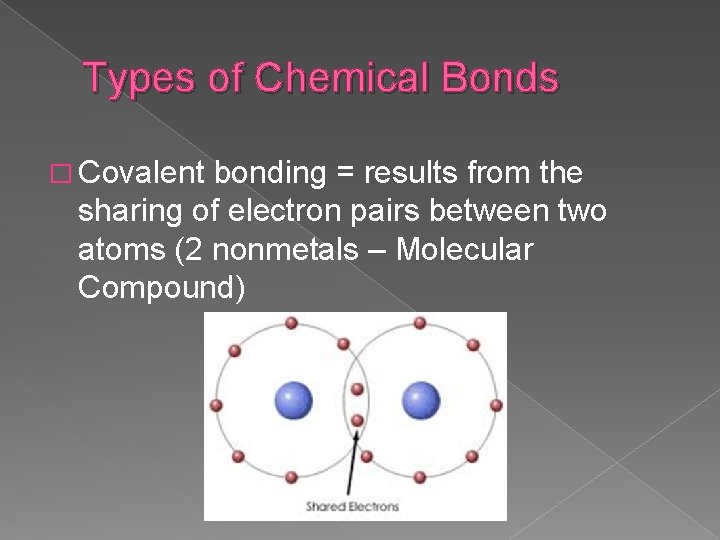
Types of Chemical Bonds � Covalent bonding = results from the sharing of electron pairs between two atoms (2 nonmetals – Molecular Compound)

Types of Chemical Bonds � Polar covalent bond = electrons are shared unequally by bonded atoms resulting in an unbalanced charge distribution
Types of Chemical Bonds � Nonpolar covalent bond = electrons are shared equally by the bonded atoms, resulting in balanced distribution of charge
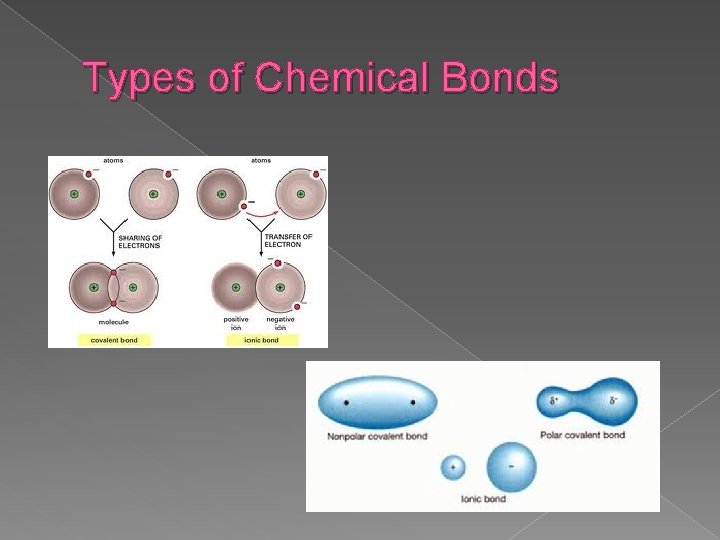
Types of Chemical Bonds
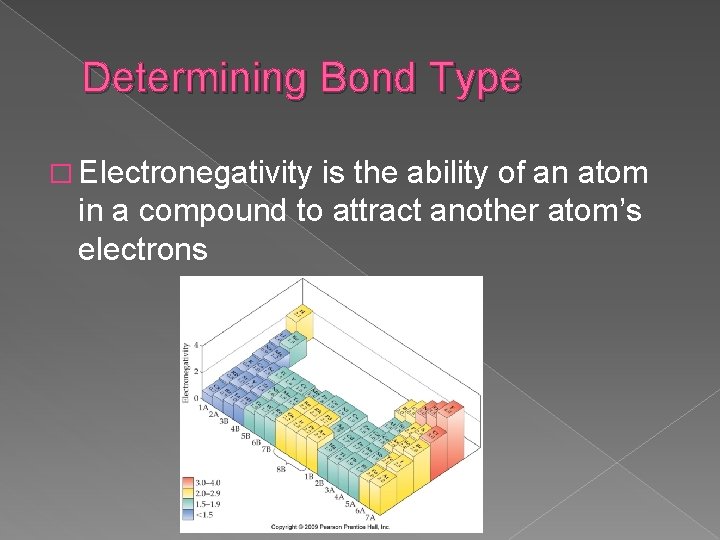
Determining Bond Type � Electronegativity is the ability of an atom in a compound to attract another atom’s electrons
Determining Bond Type � Bond type can be estimated by calculating the difference in elements’ electronegativities � Ionic bonds: 1. 71 -3. 30 � Polar covalent: 0. 31 – 1. 70 � Nonpolar covalent: 0 -0. 30

Practice Problem �Use the electronegativity differences to determine bonding in the following elements �H and S , Ca and Cl, I and I

More Practice Problems � Use the electronegativity differences to determine bonding in the following compounds; then describe each bond type. �Cl and Br, Cs and S, and P and O
Valence Electrons � the electrons in the highest occupied energy level of an element’s atoms � determine the properties of elements � equals the group number � used in bonding
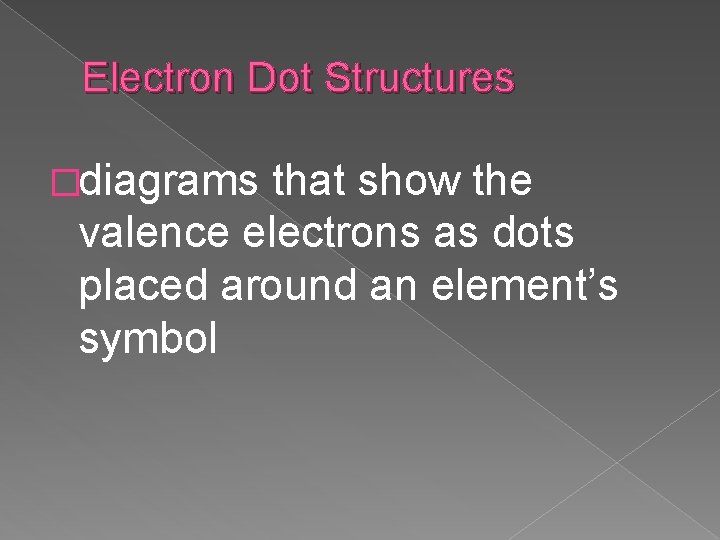
Electron Dot Structures �diagrams that show the valence electrons as dots placed around an element’s symbol
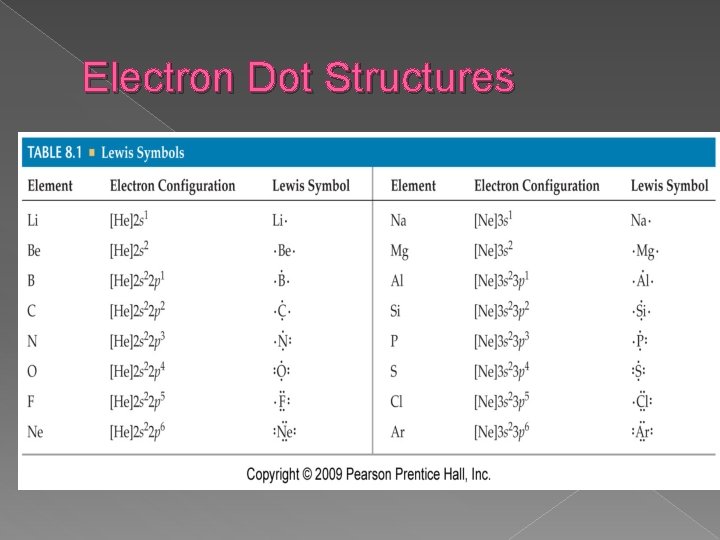
Electron Dot Structures
Octet Rule � atoms bond achieve electron configurations of a noble gas, ns 2 np 6…a set of 8 electrons to become stable
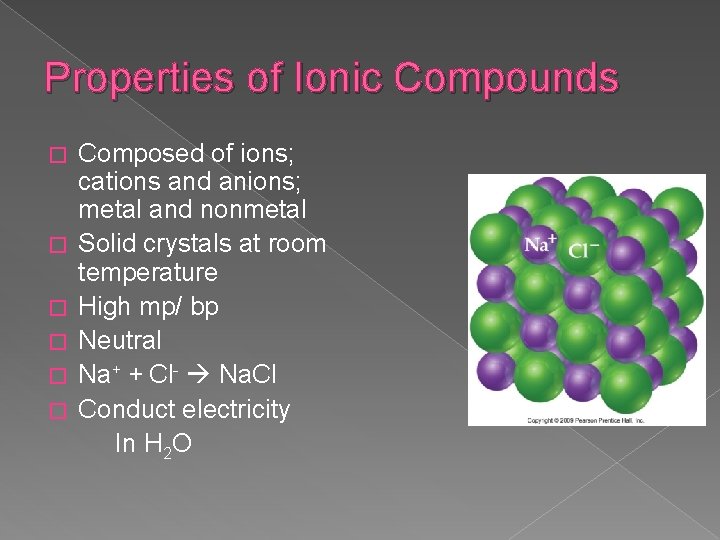
Properties of Ionic Compounds � � � Composed of ions; cations and anions; metal and nonmetal Solid crystals at room temperature High mp/ bp Neutral Na+ + Cl- Na. Cl Conduct electricity In H 2 O
Formation of Binary Ionic Compounds � 1. Draw Electron Dot Structure � 2. Show give/take of e� 3. State what happened � 4. Write the formula
Formation of Ionic Compounds �Show the formation of the bond between �Na and Br �Ca and Cl �Al and S

Formation of Ionic Compounds �Show the formation of the bond between �Al + Br, K + O, Mg + N, Li + I, Ca +P
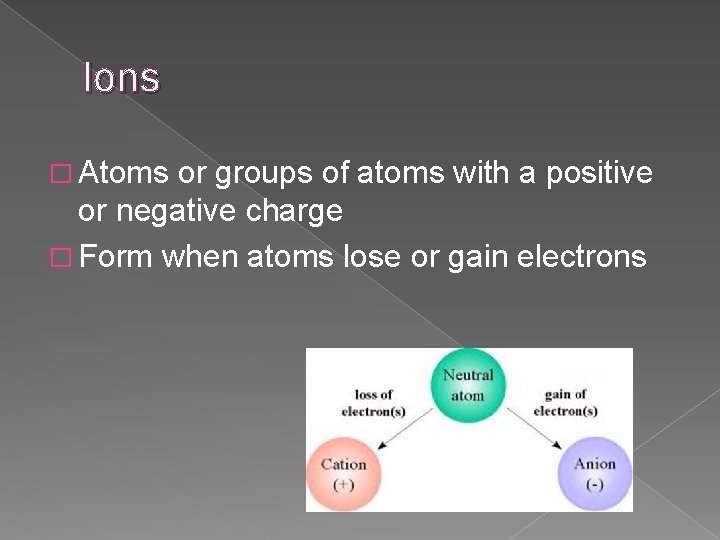
Ions � Atoms or groups of atoms with a positive or negative charge � Form when atoms lose or gain electrons


Cations � Any atom or group of atoms with a positive charge � It has lost electrons � Metals � Formula - symbol and charge � Name – elements name
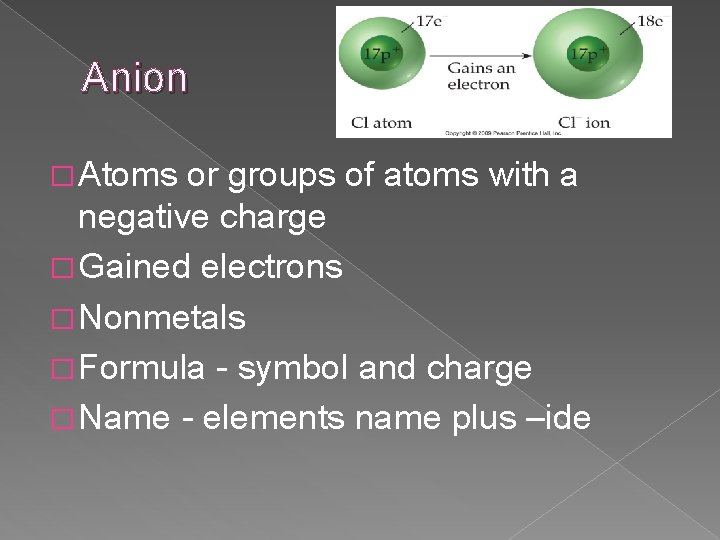
Anion � Atoms or groups of atoms with a negative charge � Gained electrons � Nonmetals � Formula - symbol and charge � Name - elements name plus –ide
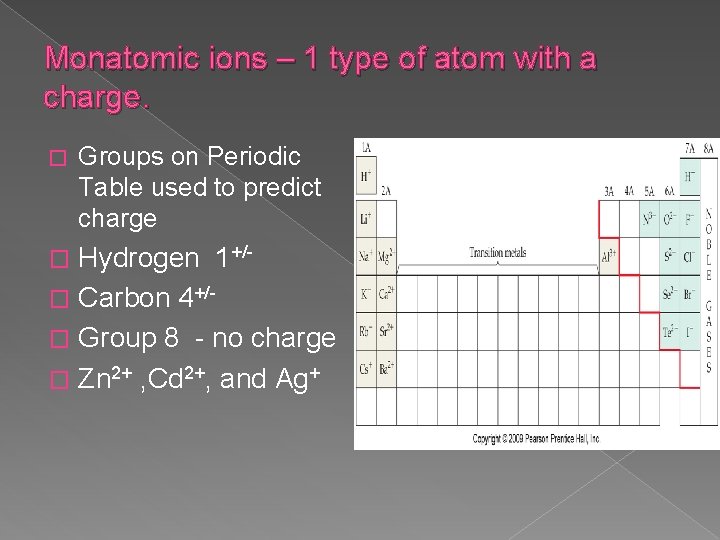
Monatomic ions – 1 type of atom with a charge. � Groups on Periodic Table used to predict charge Hydrogen 1+/� Carbon 4+/� Group 8 - no charge � Zn 2+ , Cd 2+, and Ag+ �
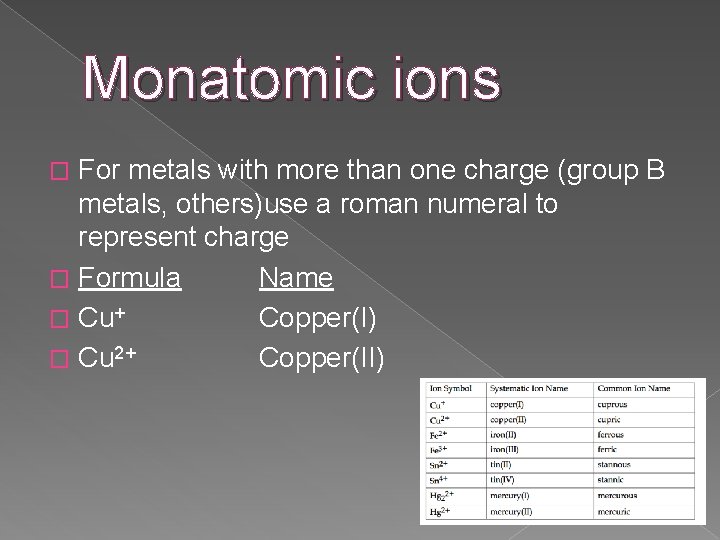
Monatomic ions For metals with more than one charge (group B metals, others)use a roman numeral to represent charge � Formula Name � Cu+ Copper(I) � Cu 2+ Copper(II) �
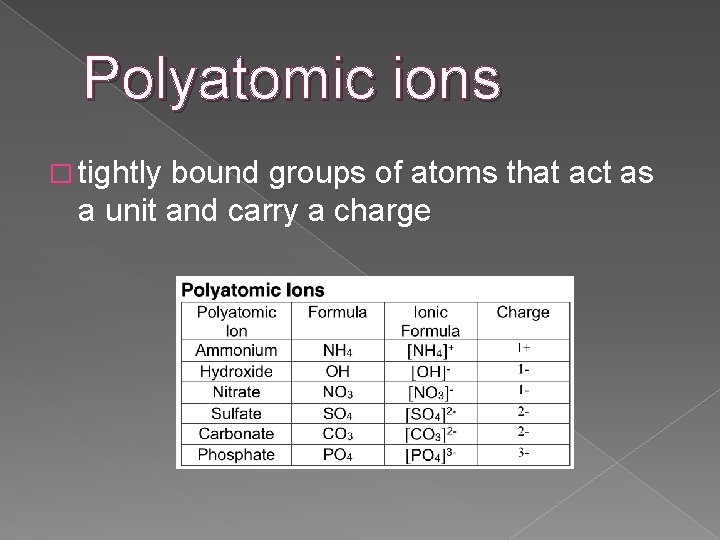
Polyatomic ions � tightly bound groups of atoms that act as a unit and carry a charge

Review: What is the name and charge for each of the following ions? �K � NH 4 � Mg � PO 4 �O � Cl. O �F � OH � Zn � SO 4

Review �What type of ions form from metals? Nonmetals? �What is the difference between Cu , Cu+, and Cu 2+? �What is an ionic compound?
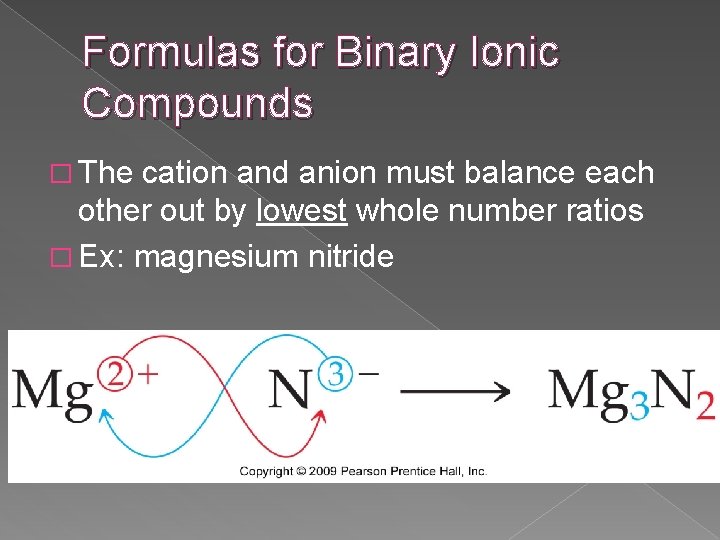
Formulas for Binary Ionic Compounds � The cation and anion must balance each other out by lowest whole number ratios � Ex: magnesium nitride

Formulas for Binary Ionic Compounds � Examples: � Potassium chloride � Calcium bromide � Iron (III) oxide � Barium sulfide

Names for Binary Ionic Compounds � Name the cation and the anion � If it is a metal with more than one charge, don’t forget the roman numeral Examples: � Mg. Cl 2 � Cu. O � Cu 2 O � Sn. O 2 �
Ternary Ionic Compounds � Same rules as binary ionic compounds except you have a polyatomic ion � Examples: � Li 3 PO 4 � Fe(OH)3 � NH 4 NO 3 � Sodium carbonate � Strontium sulfate � Iron (II) nitrate
- Slides: 38