Unit 5 Ionic Bonding Valence Electrons es in
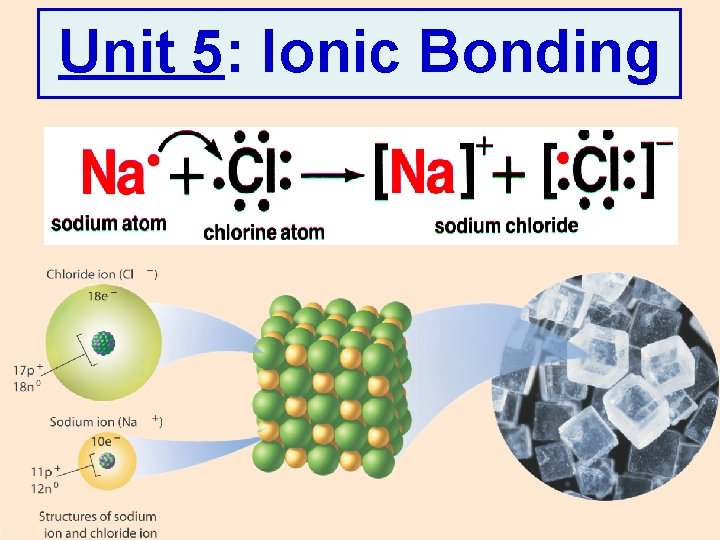
Unit 5: Ionic Bonding
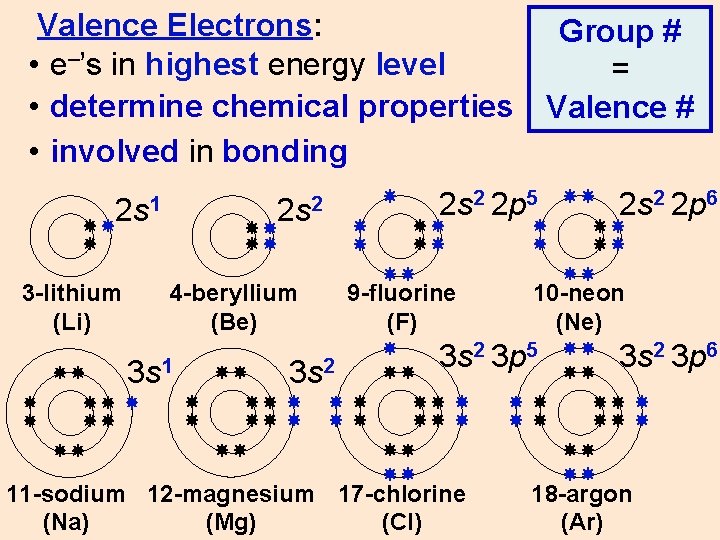
Valence Electrons: • e–’s in highest energy level • determine chemical properties • involved in bonding 2 s 1 3 -lithium (Li) 2 s 2 4 -beryllium (Be) 3 s 1 3 s 2 Group # = Valence # 2 s 2 2 p 5 9 -fluorine (F) 10 -neon (Ne) 3 s 2 3 p 5 11 -sodium 12 -magnesium 17 -chlorine (Na) (Mg) (Cl) 2 s 2 2 p 6 3 s 2 3 p 6 18 -argon (Ar)
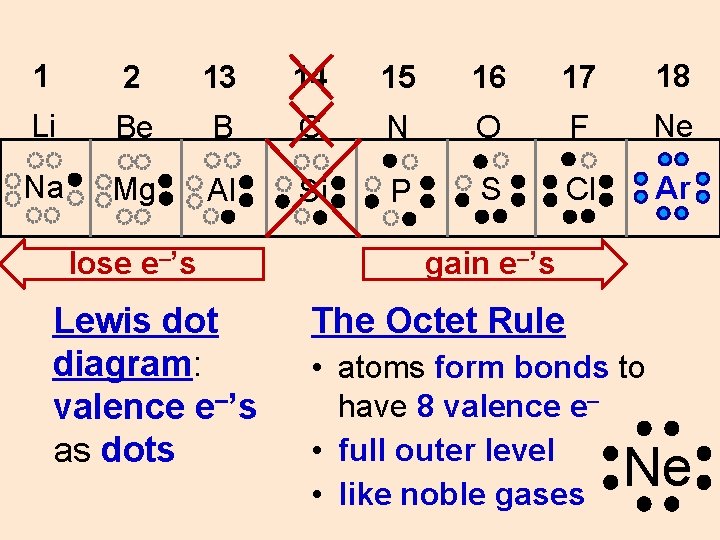
1 2 13 14 15 16 17 18 Li Be B C N O F Ne Na Mg Al Si P S Cl Ar lose e–’s Lewis dot diagram: valence e–’s as dots gain e–’s The Octet Rule • atoms form bonds to have 8 valence e– • full outer level • like noble gases Ne
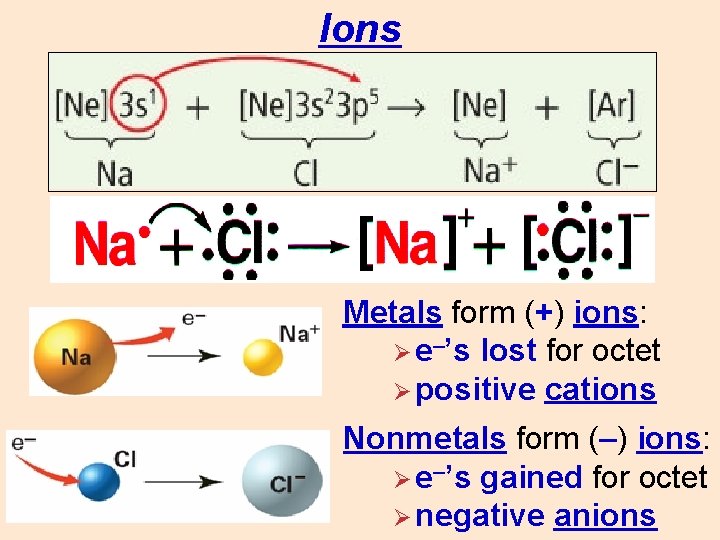
Ions Metals form (+) ions: Ø e–’s lost for octet Ø positive cations Nonmetals form (–) ions: Ø e–’s gained for octet Ø negative anions
![Group 1 1+ charged ions Na + [Na] Group 2 2+ charged ions Mg Group 1 1+ charged ions Na + [Na] Group 2 2+ charged ions Mg](http://slidetodoc.com/presentation_image_h2/1f48c8dc4598832ff73fb282fa5cd653/image-5.jpg)
Group 1 1+ charged ions Na + [Na] Group 2 2+ charged ions Mg 2+ [Mg] Group 3 A 3+ charged ions Al 3+ [Al]
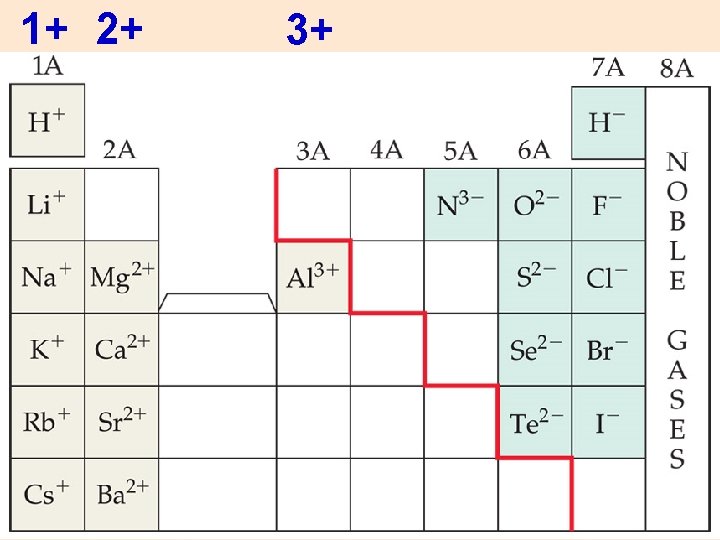
1+ 2+ 3+
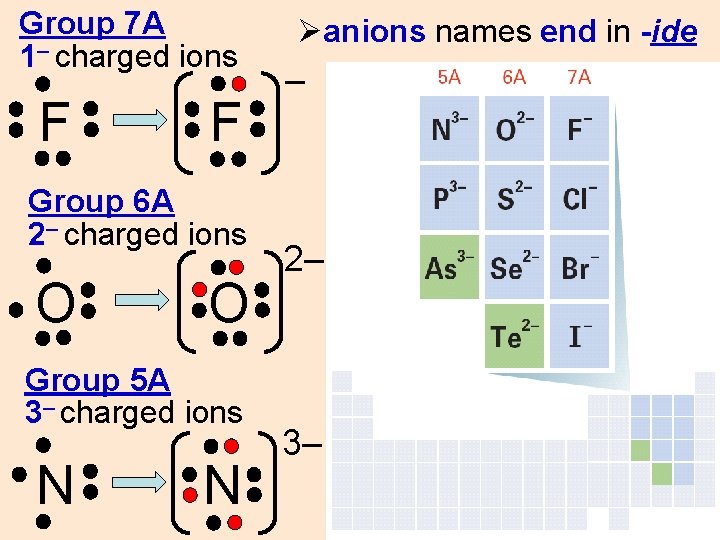
Group 7 A 1– charged ions F F Group 6 A 2– charged ions O O Group 5 A 3– charged ions N N Øanions names end in -ide – 2– 3–
![Lewis dot diagrams + [Na] Al. Cl 3 Cl – Lewis dot diagrams + [Na] Al. Cl 3 Cl –](http://slidetodoc.com/presentation_image_h2/1f48c8dc4598832ff73fb282fa5cd653/image-8.jpg)
Lewis dot diagrams + [Na] Al. Cl 3 Cl –
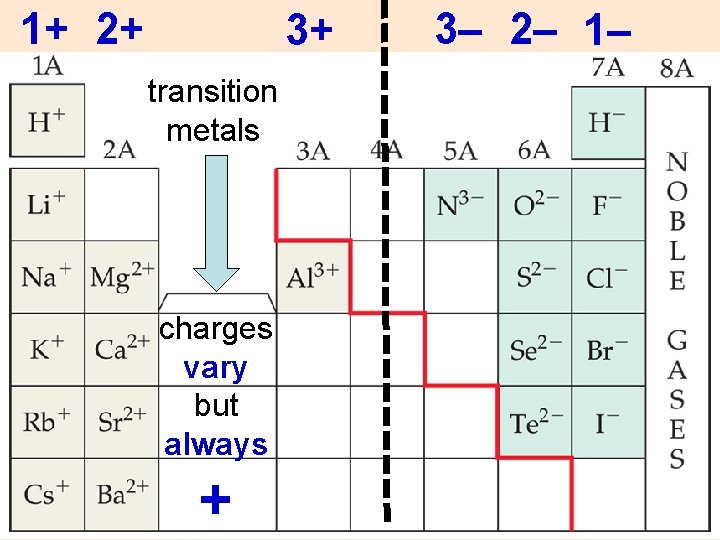
1+ 2+ 3+ transition metals charges vary but always + 3– 2– 1–
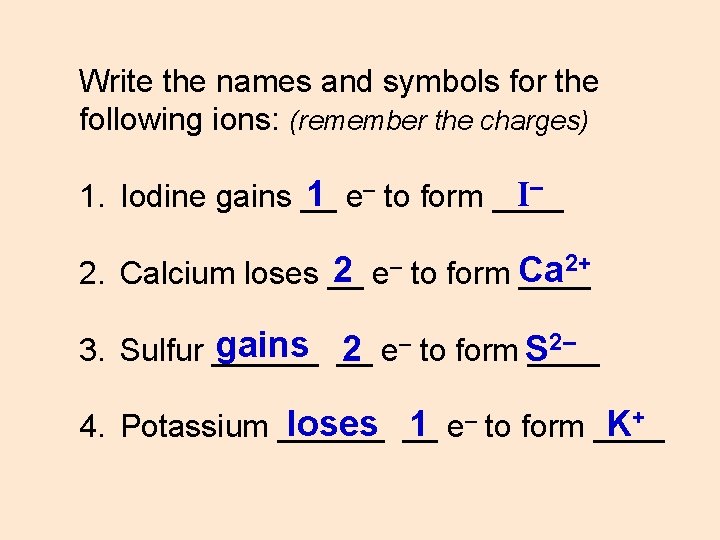
Write the names and symbols for the following ions: (remember the charges) I– 1 e– to form ____ 1. Iodine gains __ 2+ 2 e– to form Ca 2. Calcium loses __ ____ 2– gains __ 3. Sulfur ______ 2 e– to form S loses __ 1 e– to form ____ K+ 4. Potassium ______

Quick Quiz! 1. How does oxygen display the octet rule when forming an ion? A. loses 2 e–’s for a – 2 charged anion B. loses 6 e–’s for a +6 charged cation C. gains 2 e–’s for a – 2 charged anion D. gains 2 e–’s for a +2 charged cation

Quick Quiz. 2. Atoms that tend to gain a noble gas electron configuration by losing valence electrons are… A. metals B. nonmetals C. halogens D. anions

Quick Quiz. 3. When a bromine atom forms an anion, it does so by… A. losing two electrons. B. gaining two electrons. C. losing one electron. D. gaining one electron

Quick Quiz. 4. Write the name and the symbol of the ion formed from a nitrogen atom. A. nitrogen ion, N 3+ B. nitride, N– C. nitride, N 3– D. nitrogen ion, N 5–
- Slides: 14