UNIT 5 Covalent Structures How are the chemical












- Slides: 45

UNIT 5: Covalent Structures *How are the chemical formulas and chemical names written for covalent molecules? *How do you draw VSEPR diagrams for covalent compounds? *What are the names and bonding/lone pairs for each molecular shape?

COVALENT MOLECULES EQ: How are the chemical formulas and chemical names written for covalent molecules?

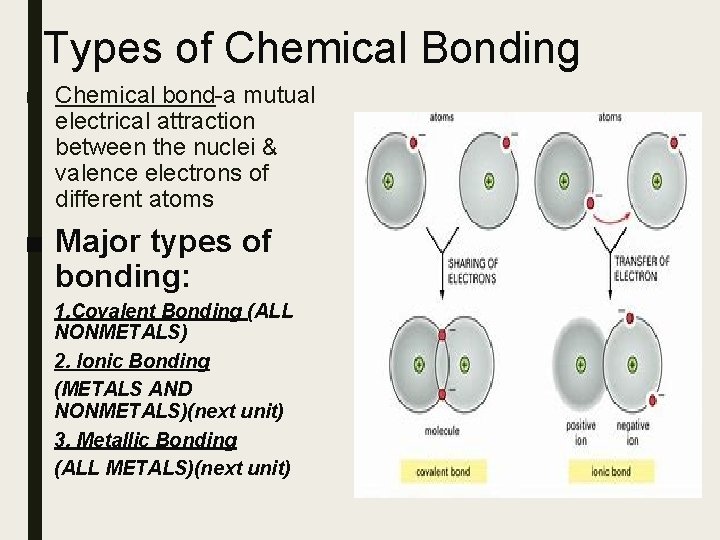
Types of Chemical Bonding ■ Chemical bond-a mutual electrical attraction between the nuclei & valence electrons of different atoms ■ Major types of bonding: 1. Covalent Bonding (ALL NONMETALS) 2. Ionic Bonding (METALS AND NONMETALS)(next unit) 3. Metallic Bonding (ALL METALS)(next unit)

Octet Rule ■ Why do atoms bond? All atoms want to be a noble gas. Why? – Noble gases have a full outer energy level. This gives great stability! ■ When atoms bond, they gain stability by filling their outer energy level through sharing or exchanging electrons. ■ Octet rule-chemical compounds tend to form so that each atom, but gaining, losing, or sharing electrons, has an octet of electrons in it highest occupied energy level.

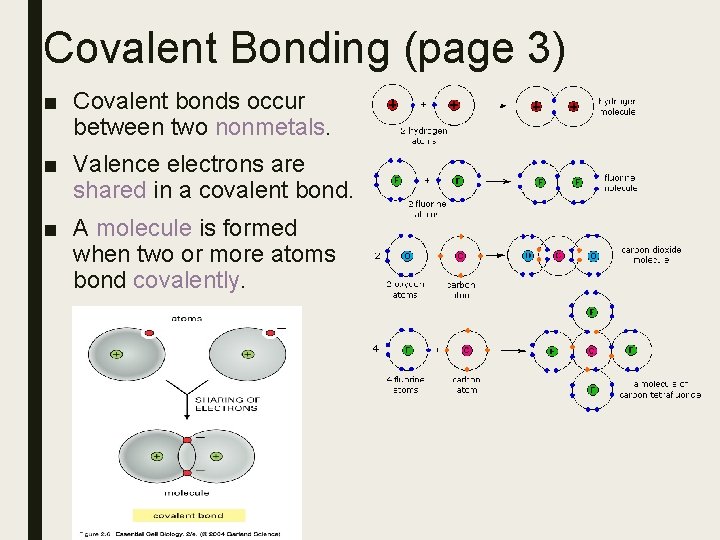
Covalent Bonding (page 3) ■ Covalent bonds occur between two nonmetals. ■ Valence electrons are shared in a covalent bond. ■ A molecule is formed when two or more atoms bond covalently.

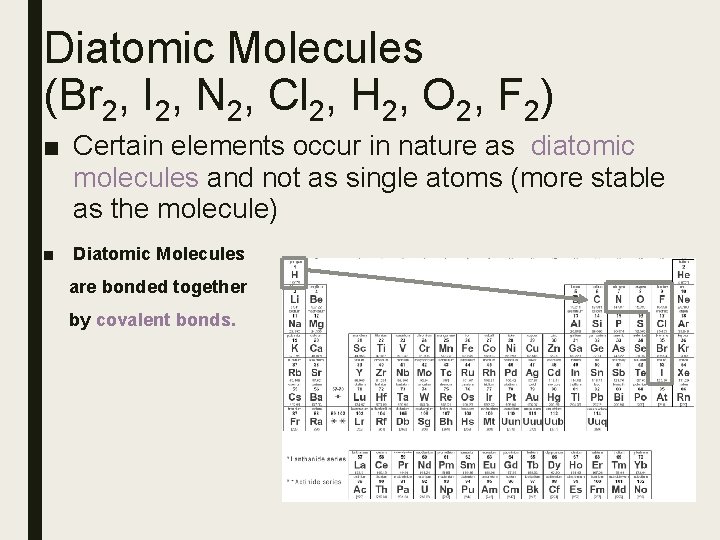
Diatomic Molecules (Br 2, I 2, N 2, Cl 2, H 2, O 2, F 2) ■ Certain elements occur in nature as diatomic molecules and not as single atoms (more stable as the molecule) ■ Diatomic Molecules are bonded together by covalent bonds.

Memorize these compound names, DO NO USE THE PREFIX ■SYSTEM: We use a prefix system to name most compounds that are covalent. BUT…you will need to know the formula and the common names for the following compounds: –Ozone - O 3 –Methane - CH 4 –Hydrogen peroxide - H 2 O 2 –Water - H 2 O –Ammonia - NH 3

HOW TO NAME COVALENT COMPOUNDS ■ The first element written in the formula is always written first, using the element name. ■ The second element is named using the root of the element and the suffix “-ide”. ■ Prefixes are used to indicate the number of atoms of each type that are present in the compound. – Exception: Do not use the prefix mono- for the first element ■ Drop the final letter of the prefix when the element name begins with a vowel ■ When naming gases, simply use the element name and add the word gas at the end

Important Definitions ■ Chemical Formula: a representation, using symbols, showing the different types and number of atoms that make up a given compound or molecule ■ Subscript: small number, written behind an element, showing how many atoms of that element are found in a compound or molecule

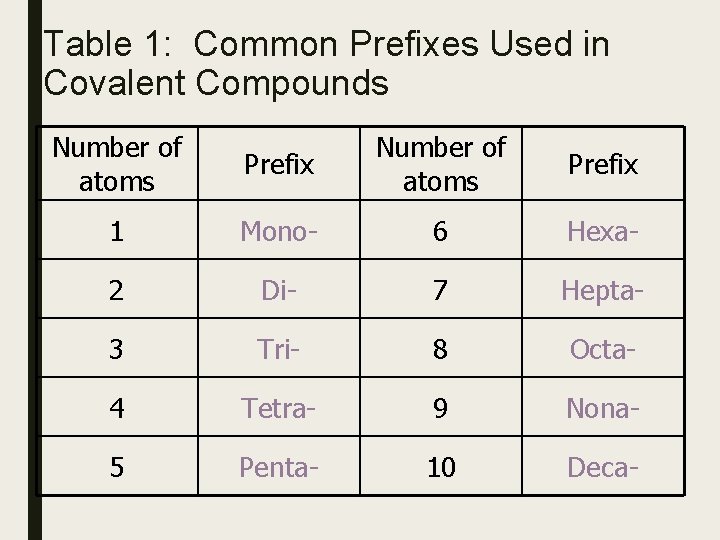
Table 1: Common Prefixes Used in Covalent Compounds Number of atoms Prefix 1 Mono- 6 Hexa- 2 Di- 7 Hepta- 3 Tri- 8 Octa- 4 Tetra- 9 Nona- 5 Penta- 10 Deca-

What Does Naming Look Like? 1. CO 2: Carbon dioxide 2. SO 2: Sulfur monoxide 3. S 2 O 3: Disulfur trifluoride 4. N 2 O: Dinitrogen monoxide

Now, you practice 1. CO 2: Carbon dioxide ______________ Sulfur dioxide 2. SO 2: Disulfur trioxide ______________ Dinitrogen monoxide 3. S 2 O 3: _____________ Phosphorous pentachloride 4. N 2 O: Oxygen difluoride ______________ 5. PCl 5: Dinitrogen tetroxide ______________ Tetrasulfur tetranitride 6. OF 2: ______________ Silicon tetrachloride 7. N 2 O 4: Boron _____________ trifluoride 8. S 4 N 4:

What’s the formula? 1. 2. 3. 4. 5. Se. F 6 Selenium hexafluoride: ______ Si 2 Br 6 Disilicon hexabromide: _____ B 2 Si Diboron monosilicide: _____ IF 5 Iodine pentafluoride: ____ 6 Si Hexaboron monosilicide: B ____ 7. 2 Br 4 Diboron tetrabromide: B_______ 2 O 5 Diphosphorous pentoxide: P______ 8. 4 Carbon tetrafluoride: CF ____ 6. 4 S 5 Tetraphosphorous pentasulfide: P_______ S 6 O 10. Hexasulfur monoxide: _____ 9.

Answer the following questions on the lined page in your packet 1. What are the 7 diatomic elements 2. What is a covalent compound? An ionic compound? 3. Name the following: C 5 H 9, N 2 Cl 3, Se. O 4, P 3 Cl 6, NO, F 2 4. Write the formula for the following: Trinitrogen hexabromide Nitrogen gas dinitrogen monoxide heptacarbon nonahydrife sulfur diselenide 5. Are the following ionic or covalent compounds: A. Mg. SO 4 B. Dinitrogen tetroxide C. Aluminum chloride D. SO 2 E. Two fluorine atoms

LEWIS STRUCTURES

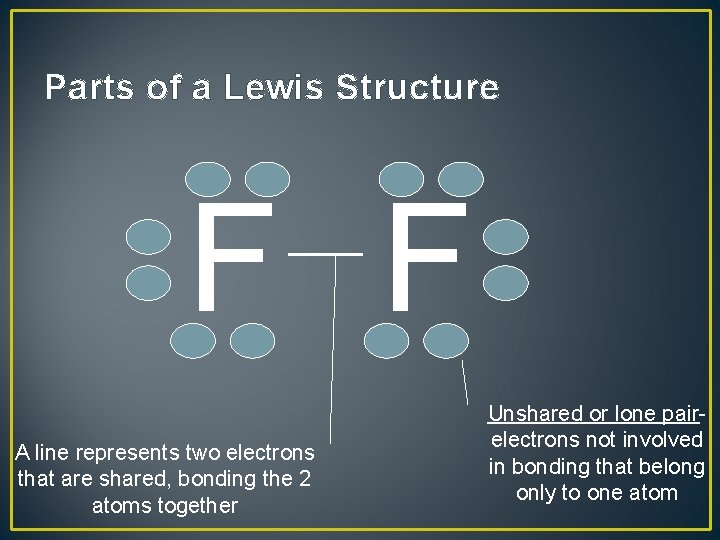
Parts of a Lewis Structure F F A line represents two electrons that are shared, bonding the 2 atoms together Unshared or lone pairelectrons not involved in bonding that belong only to one atom

NAS Method to drawing Lewis Structures • Needed, Available, Shared Method 1. Determine how many electrons are available by adding up the total number of valence electrons each atom in the molecule has. 2. Determine how many electrons are needed to satisfy each atoms’ octet. Don’t forget exceptions! 3. Subtract available from needed. This will tell you how many electrons have to be shared. 4. Divide the shared electrons by 2 to figure out how many bonds you will need.

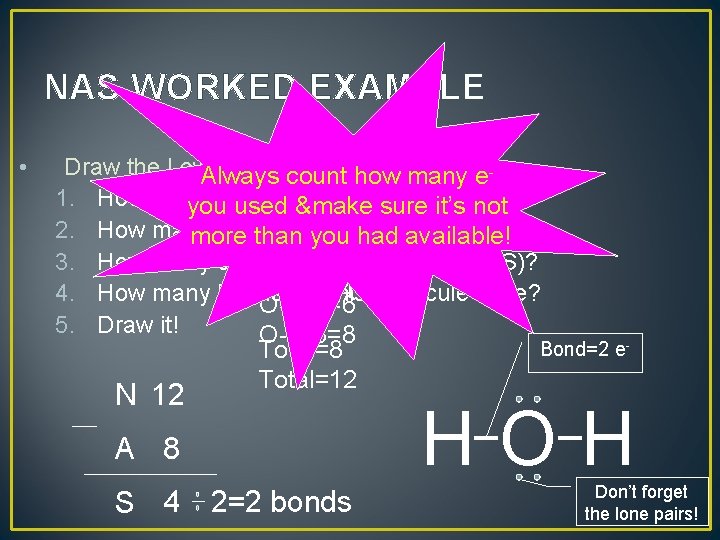
NAS WORKED EXAMPLE • Draw the Lewis structure water, H 2 O. Always countforhow many e 1. How many are available youelectrons used &make sure it’s(A)? not 2. How many electrons are had needed (N)? more than you available! H-2 x 1=2 3. How many electrons must be shared (S)? H-2 x 2=4 4. How many bonds will this molecule have? O-1 x 6=6 5. Draw it! O-1 x 8=8 Bond=2 e. Total=8 Total=12 N 12 HOH A 8 S 4 2=2 bonds Don’t forget the lone pairs!

NAS WORKED EXAMPLE • I HCH H Draw the Lewis structure for CH 3 I. 1. How many electrons are available (A)? 2. How many electrons are needed (N)? C-1 x 4=4 3. How many electrons must be shared (S)? C-1 x 8=8 4. How many bonds will this molecule have? H-3 x 1=3 5. Draw it! H-3 x 2=6 I-1 x 7=7 I-1 x 8=8 Total=14 N 22 Total=22 A 14 S 8 2=4 bonds

LET’S WORK SOME OUT Page 5 #1 -5

LEWIS STRUCTURE EXAMPLE #1 • CCl 4

LEWIS STRUCTURE EXAMPLE #2 • H 2 CS

LEWIS STRUCTURE EXAMPLE #3 NI 3

LEWIS STRUCTURE EXAMPLE #4 • Sulfate

LEWIS STRUCTURE EXAMPLE #5 • C 2 H 2

LEWIS STRUCTURE EXAMPLE #6 • NH 3

LEWIS STRUCTURE EXAMPLE #7 • Carbonate

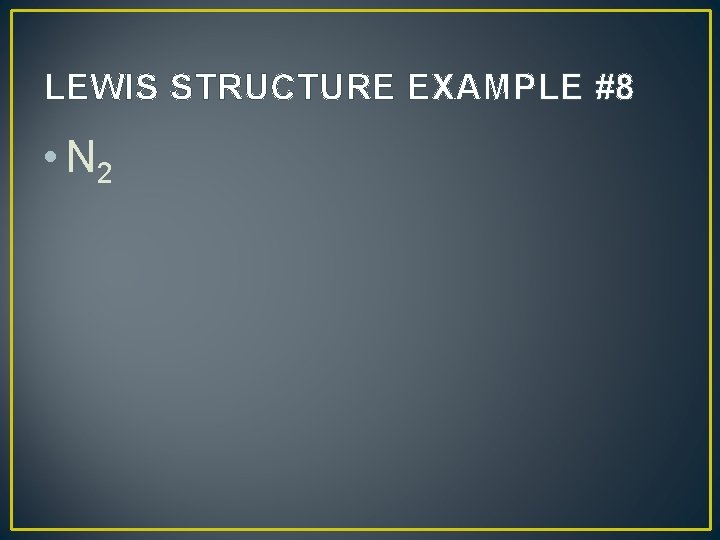
LEWIS STRUCTURE EXAMPLE #8 • N 2

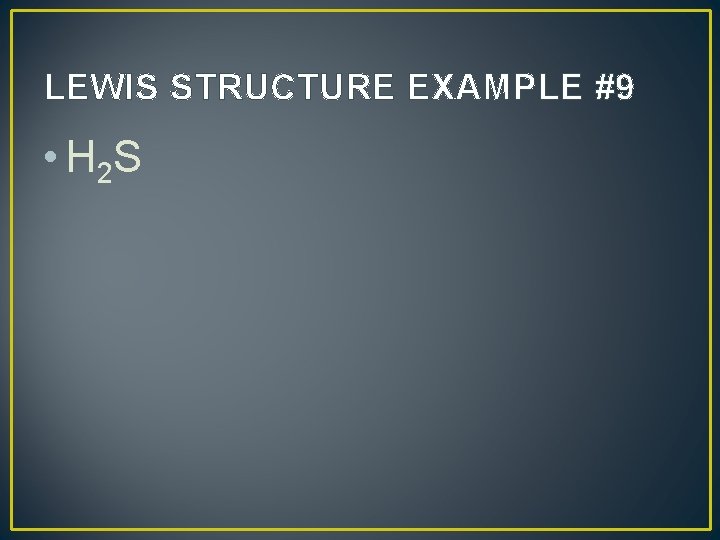
LEWIS STRUCTURE EXAMPLE #9 • H 2 S

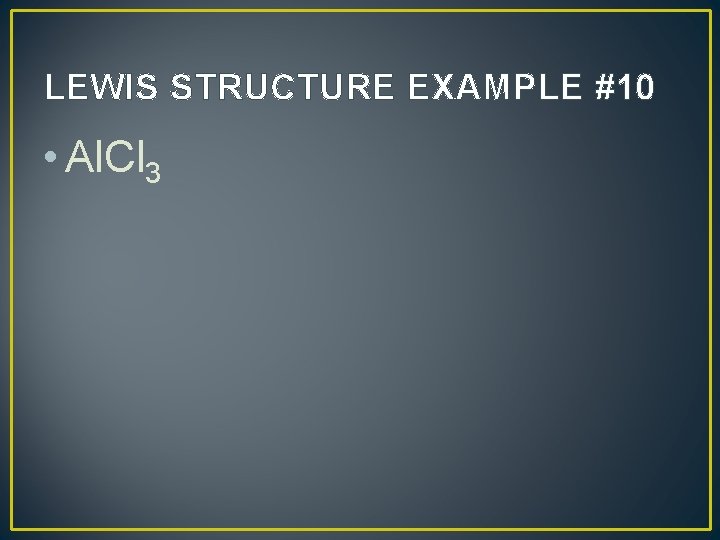
LEWIS STRUCTURE EXAMPLE #10 • Al. Cl 3

Molecular Geometry TAKE NOTES ON PAGE 8

VSEPR Theory (molecular shape) • “Valence Shell Electron Pair Repulsion” Theory • Electron pairs orient themselves in order to minimize repulsive forces.

VSEPR Theory Vocab • Types of e- Pairs • Bonding pairs - form bonds • Lone pairs - nonbonding electrons (these will DETERMINE THE SHAPE MOST OF THE TIME IF THERE ARE LONE PAIRS ON THE CENTRAL ATOM: BENDS THE MOLECULE) • Molecular geometry-shape the molecule takes on to minimize the repulsion between its electrons.

Steps to Determine Molecular Shape 1. Draw the Lewis Diagram. 2. Look at atom in the center to see if there are lone pairs 3. Count how many atoms are bonded to central atom Know the 5 common shapes

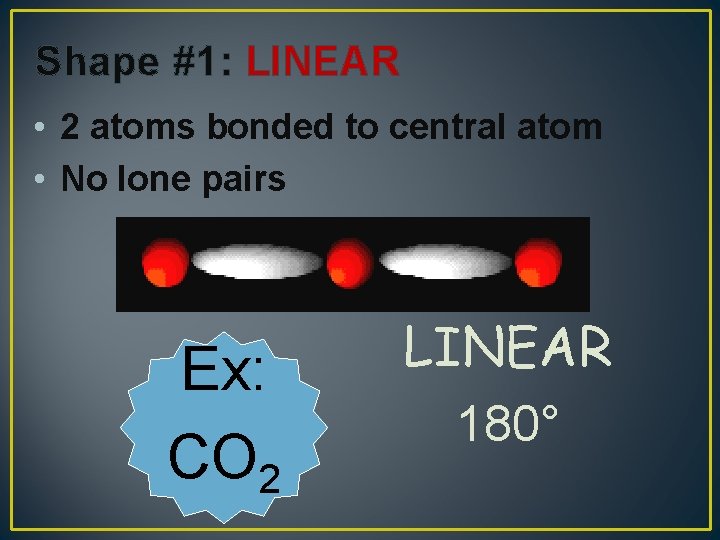
Shape #1: LINEAR • 2 atoms bonded to central atom • No lone pairs Ex: CO 2 LINEAR 180°

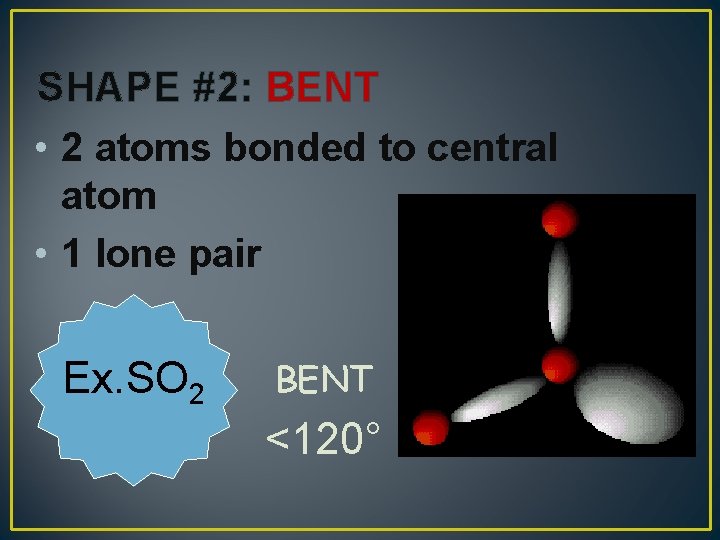
SHAPE #2: BENT • 2 atoms bonded to central atom • 1 lone pair Ex. SO 2 BENT <120°

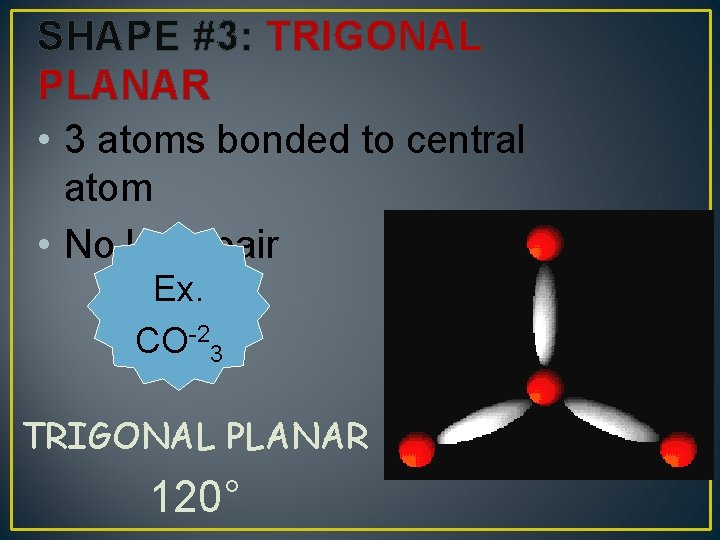
SHAPE #3: TRIGONAL PLANAR • 3 atoms bonded to central atom • No lone pair Ex. CO-23 TRIGONAL PLANAR 120°

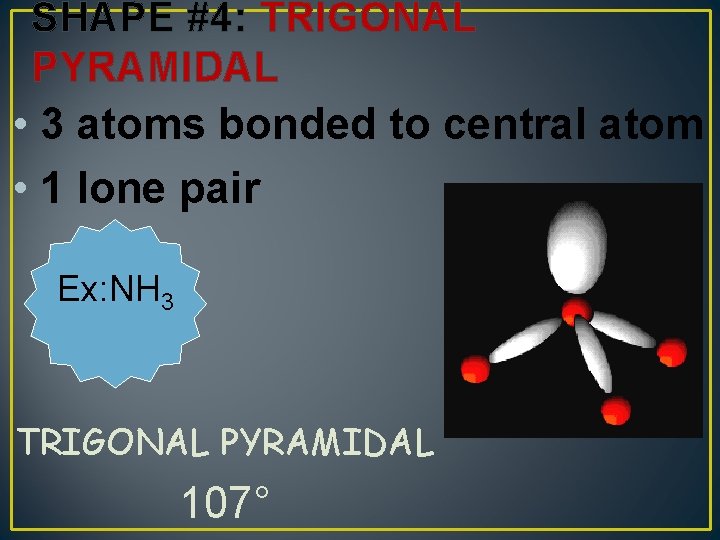
SHAPE #4: TRIGONAL PYRAMIDAL • 3 atoms bonded to central atom • 1 lone pair Ex: NH 3 TRIGONAL PYRAMIDAL 107°

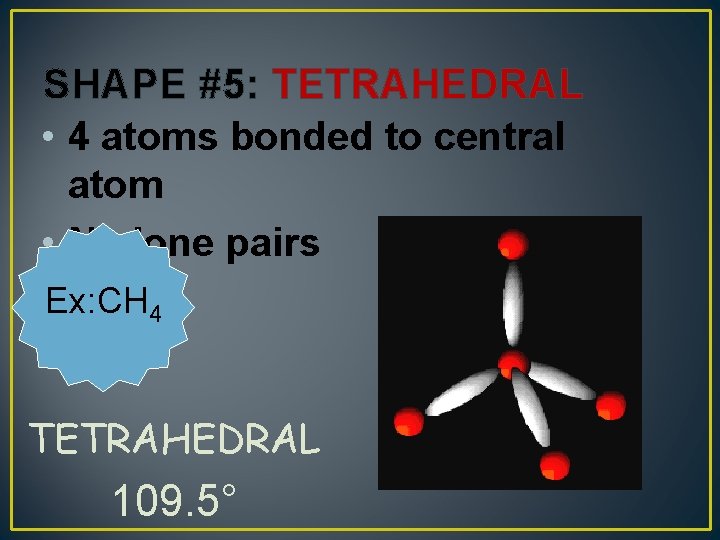
SHAPE #5: TETRAHEDRAL • 4 atoms bonded to central atom • No lone pairs Ex: CH 4 TETRAHEDRAL 109. 5°

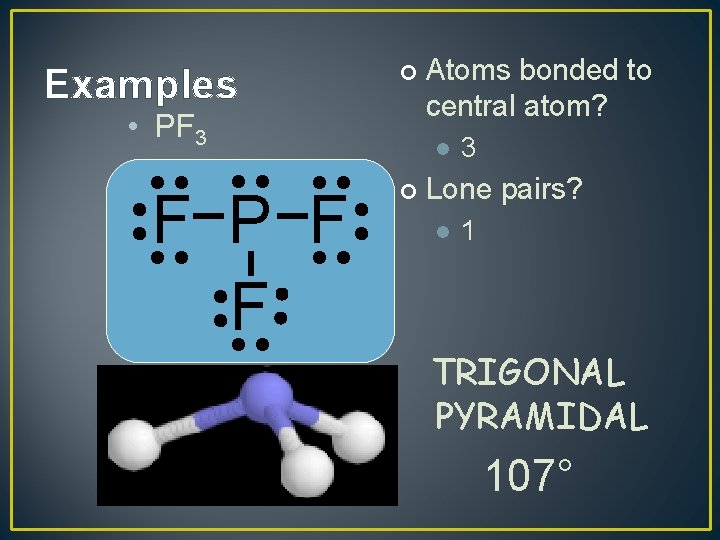
Examples ¢ • PF 3 F P F F Atoms bonded to central atom? l ¢ 3 Lone pairs? l 1 TRIGONAL PYRAMIDAL 107°

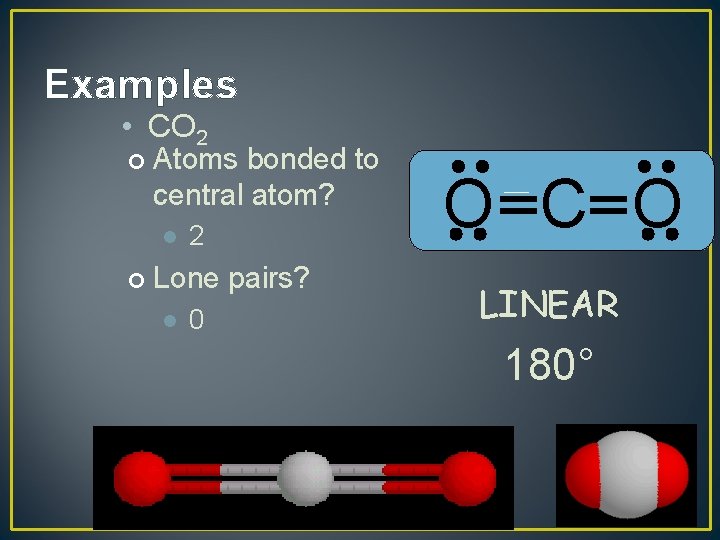
Examples • CO 2 ¢ Atoms bonded to central atom? l ¢ 2 Lone pairs? l 0 O C O LINEAR 180°

POLARITY

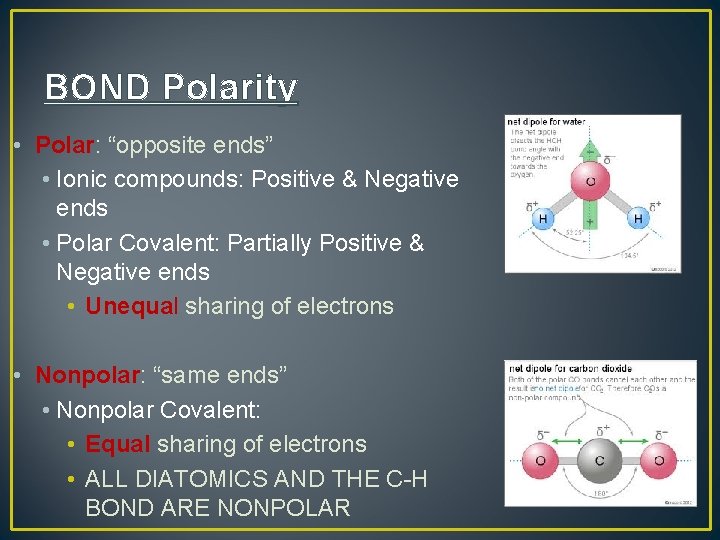
BOND Polarity • Polar: “opposite ends” • Ionic compounds: Positive & Negative ends • Polar Covalent: Partially Positive & Negative ends • Unequal sharing of electrons • Nonpolar: “same ends” • Nonpolar Covalent: • Equal sharing of electrons • ALL DIATOMICS AND THE C-H BOND ARE NONPOLAR

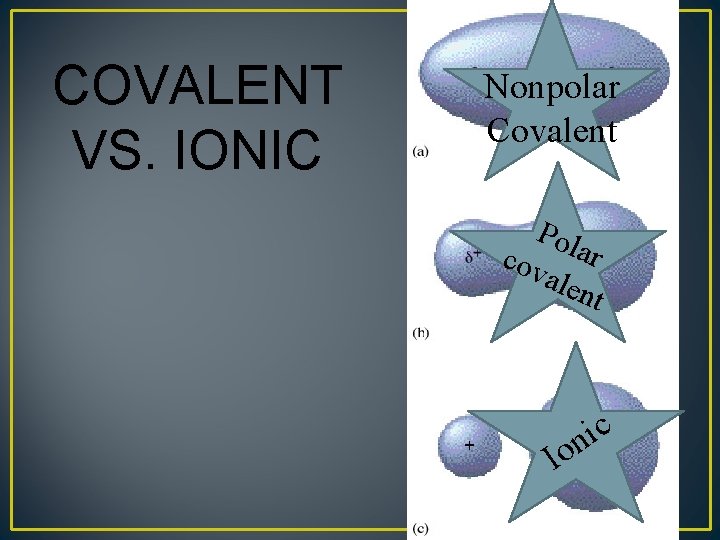
COVALENT VS. IONIC Nonpolar Covalent Pol cov ar alen t c i n o I

POLARITY OF MOLECULES • IF THERE ARE NO LONE PAIRS ON THE CENTRAL ATOM, THE MOLECULE IS NONPOLAR • LINEAR • TRIGONAL PLANAR • TETRAHEDRAL • IF THERE ARE LONE PAIRS ON THE CENTRAL ATOM, THE MOLECULE IS POLAR: • BENT • TRIGONAL PYRAMIDAL