UNIT 5 CHEMICAL NAMING BALANCING Chapter 6 15

UNIT 5 CHEMICAL NAMING & BALANCING Chapter 6, 15. 1, 16. 1

NOMENCLATURE: PROCESS USED FOR NAMING CHEMICAL COMPOUNDS n n Atoms of different elements combine to form compounds that are represented by chemical formulas. All compounds can be named in such a way that others can understand the make-up of the compound.
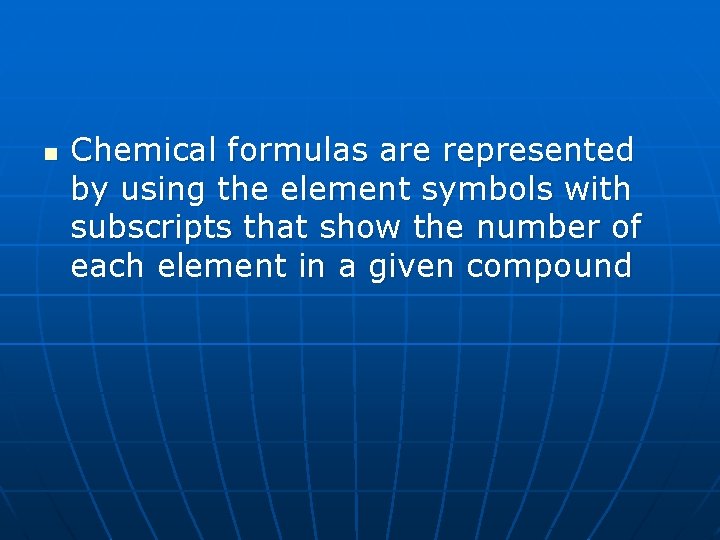
n Chemical formulas are represented by using the element symbols with subscripts that show the number of each element in a given compound
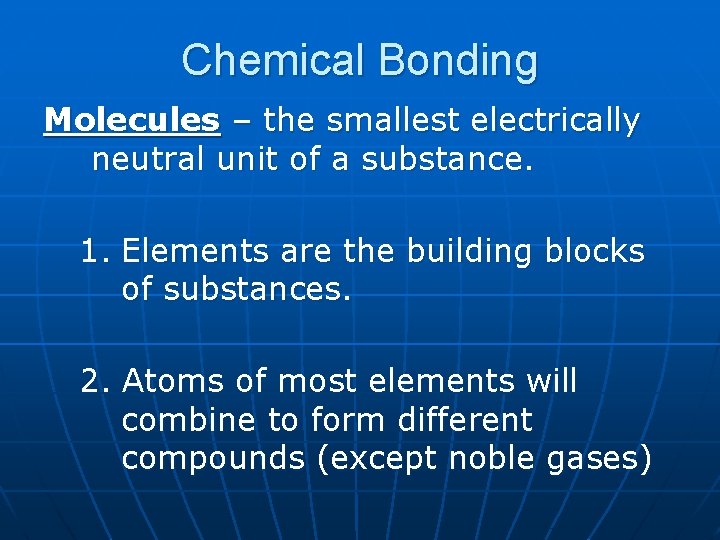
Chemical Bonding Molecules – the smallest electrically neutral unit of a substance. 1. Elements are the building blocks of substances. 2. Atoms of most elements will combine to form different compounds (except noble gases)

3. Molecules are made up of 2 or more atoms that act as one unit. Ex: O 2, O 3, H 2 O, CO 2 4. Molecular compounds can be called Diatomic, or Triatomic molecules. 5. Molecular (Binary) Compounds are composed of molecules that are two or more non-metals.

Ions & Ionic Compounds 1. Ions are atoms or a group of atoms that have lost or gained electrons 2. A loss of electrons gives the atom a positive charge, therefore a gain of an electron would give the atom a negative charge.
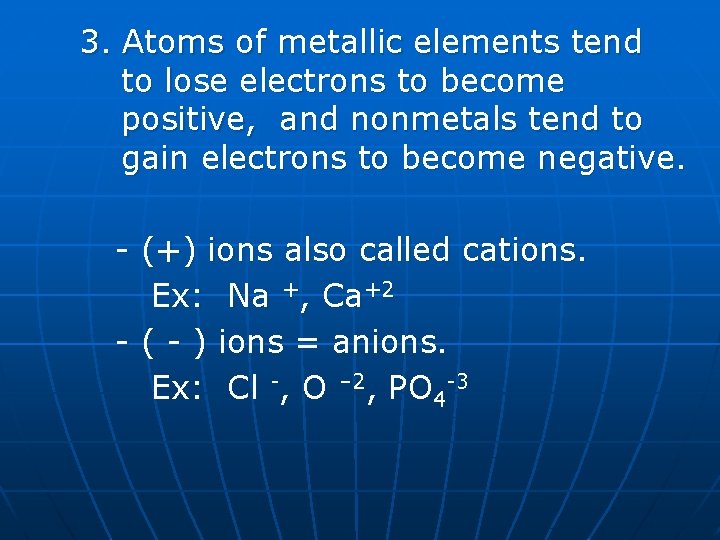
3. Atoms of metallic elements tend to lose electrons to become positive, and nonmetals tend to gain electrons to become negative. - (+) ions also called cations. Ex: Na +, Ca+2 - ( - ) ions = anions. Ex: Cl -, O – 2, PO 4 -3

4. Ionic compounds consist of a cation bonded to an anion. The charge of the compound is neutral.

OXIDATION NUMBER • This is a positive or negative number assigned to an atom according to a set of rules The oxidation number of a monatomic ion is equal to its ionic charge Ex. Br- = - 1 Mg +2 = +2

• • • The oxidation number of hydrogen in a compound is +1 except in a metal hydride where it is -1 The oxidation number of oxygen is -2 except in peroxides where it is -1 The oxidation number of the atom in its elemental form is 0
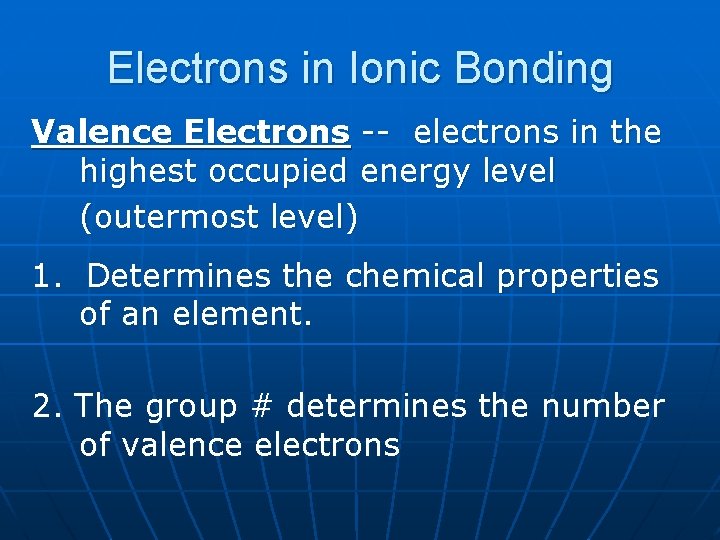
Electrons in Ionic Bonding Valence Electrons -- electrons in the highest occupied energy level (outermost level) 1. Determines the chemical properties of an element. 2. The group # determines the number of valence electrons
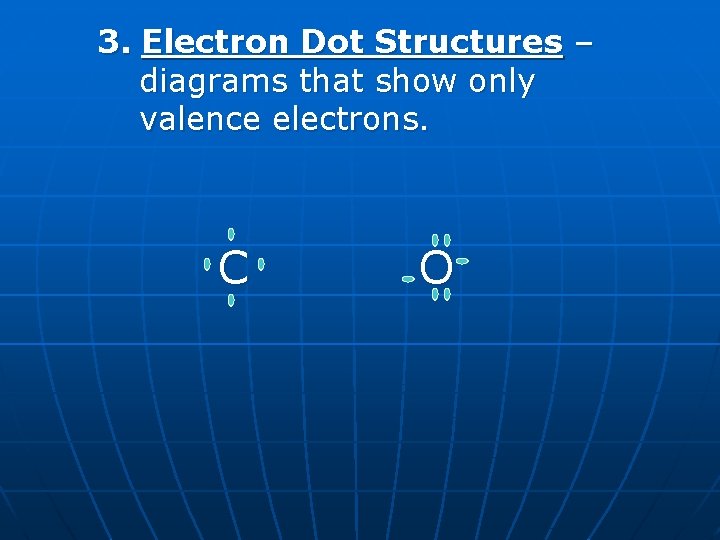
3. Electron Dot Structures – diagrams that show only valence electrons. C O

Octet rule -- atoms tend to achieve the electron configuration of a noble gas. Atoms will gain or lose electrons to achieve this. Ionic Bonds -- the force of attraction between two ions. (Metal & Nonmetal)
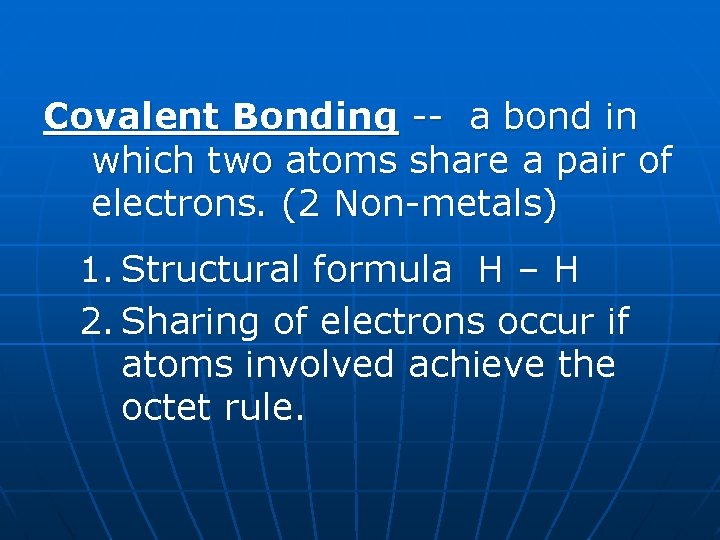
Covalent Bonding -- a bond in which two atoms share a pair of electrons. (2 Non-metals) 1. Structural formula H – H 2. Sharing of electrons occur if atoms involved achieve the octet rule.
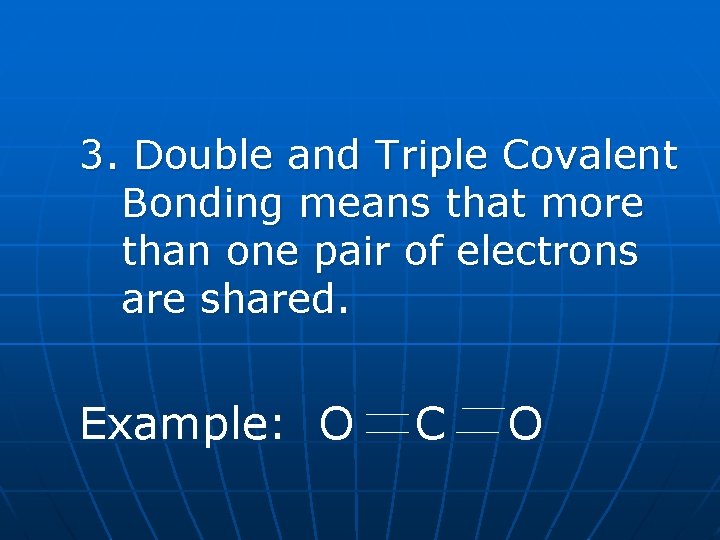
3. Double and Triple Covalent Bonding means that more than one pair of electrons are shared. Example: O C O
Chemical Compounds Chemical Formula’s -- Shows the types and numbers of atoms in a compound. - Subscripts show the number of atoms. Ex: H 2 O
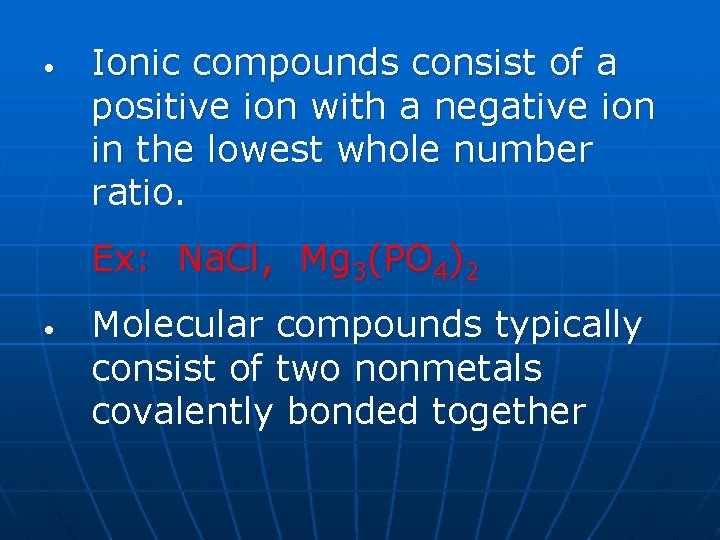
• Ionic compounds consist of a positive ion with a negative ion in the lowest whole number ratio. Ex: Na. Cl, Mg 3(PO 4)2 • Molecular compounds typically consist of two nonmetals covalently bonded together

Ionic Charges • • Monoatomic ions consist of only 1 ion. Polyatomic ions consist of tightly bound atoms that behave as a single unit, that has either lost or gained electrons to become (+) or (-). Ex: NO 3 -, SO 4 -2

Balancing Formulas

Writing Formulas Ionic Compounds • Criss Cross Method 1. Write symbol and charge for each ion present.

Compare charges: • If charges equal 0 then simply write symbols together. • If charges don’t equal 0 then cross charges and write them as a subscript.
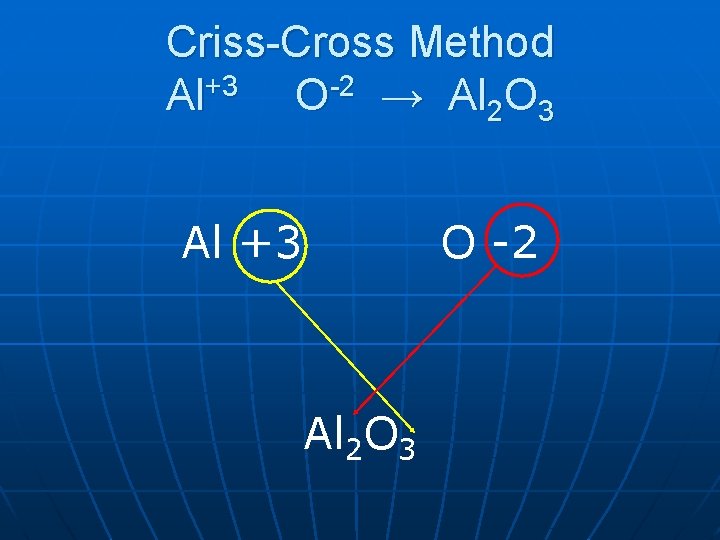
Criss-Cross Method Al+3 O-2 → Al 2 O 3 Al +3 O -2 Al 2 O 3
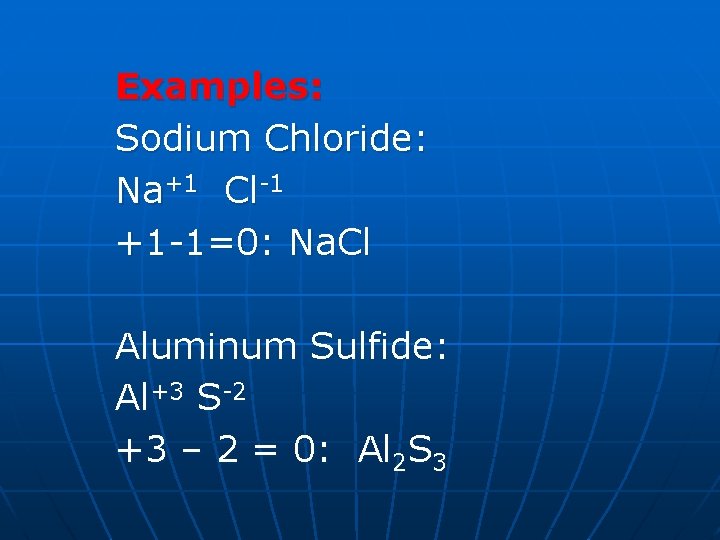
Examples: Sodium Chloride: Na+1 Cl-1 +1 -1=0: Na. Cl Aluminum Sulfide: Al+3 S-2 +3 – 2 = 0: Al 2 S 3
Binary Molecular Compounds Consists of two nonmetals 1. Write the symbols of the two elements, change prefixes to subscripts.
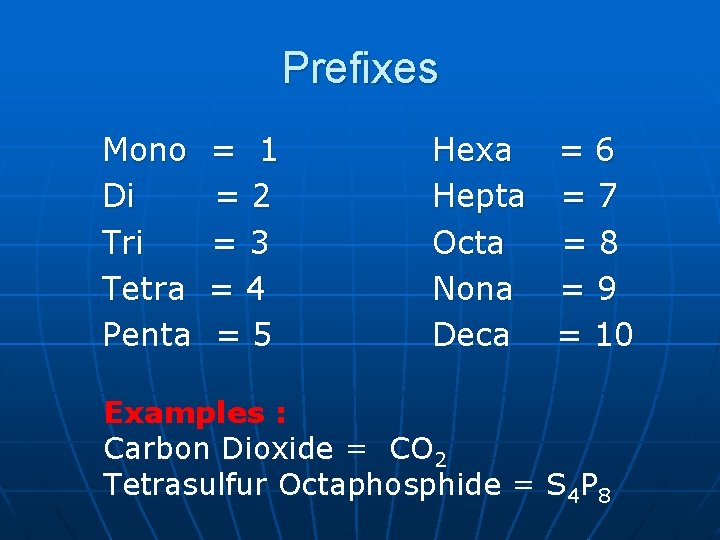
Prefixes Mono Di Tri Tetra Penta = 1 =2 =3 =4 =5 Hexa = 6 Hepta = 7 Octa =8 Nona = 9 Deca = 10 Examples : Carbon Dioxide = CO 2 Tetrasulfur Octaphosphide = S 4 P 8

Acids 1. Acids consist of a hydrogen bonded to an anion (negative ion ). Step 1: because of the word acid in the name, place the hydrogen ion ( H+1) in the positive ion position of a compound.

Step 2: apply the “ hydro-ic-ide, ” the “ic-ate, ”or the “ous-ite” rule to determine the name of the negative ion. Examples: - Hydrochloric Acid - Nitrous Acid

Step 3: balance the electrical charges for this compound as you would when writing any chemical formula.

Naming Compounds

Naming Compounds Ionic Compounds 1. Write the names of the ions present. 2. Determine which cation forms the compound by looking at the ratio of the atoms and compare to the ratio of the charges.

• Remember that the overall charge of the compound has to equal zero. • Example: Cu. O is it copper I or II? The ratio of atoms is 1: 1 and the charge on O is – 2 so the Cu has to be 2 to have the same ratio. The name of the compound would be Copper II Oxide

Binary Molecular Compounds: 1. Write the name of the element in the first position and if it has a subscript change it to a prefix.

2. Write the name of the second element. Change the ending to –ide, and always use a prefix that corresponds with the number of atoms present in the molecule. Example : PO 5 = Phosphorus Pentaoxide S 2 N = Disulfur Mononitride

Acids **If the compound starts with a H then it considered to be an acid. Step 1: Place the word acid at the end of the compound. This takes care of naming the hydrogen ion.

Step 2: name the negative ion by applying the acid rules: - If the ending is – ide, place hydro- in front of the negative ion name, then drop the –ide and add –ic. - If the ending is –ate, drop the ate ending and add –ic. - If the ending is -ite, drop the ending and add –ous.
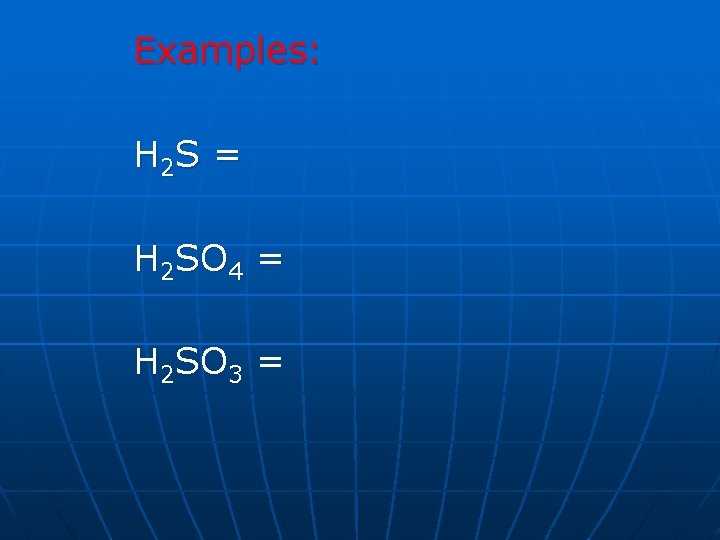
Examples: H 2 S = H 2 SO 4 = H 2 SO 3 =
MOLECULAR WEIGHTS
- Slides: 37