UNIT 4 Syllabus Solidification Mechanism of solidification Homogenous
UNIT – 4 Syllabus Solidification: Mechanism of solidification, Homogenous and Heterogeneous nucleation, crystal growth, cast metal structures. Phase Diagram I: Solid solutions Hume Rothary rule substitutional, and interstitial solid solutions, intermediate phases, Gibbs phase rule.
What is solidification? • Solidification is the process where liquid metal transforms into solid upon cooling • The structure produced by solidification, particularly the grain size and grain shape, affects to a large extent the properties of the products • At any temp, thermodynamically stable state is the one which has the lowest free energy and consequently, any other state tends to change the stable form.
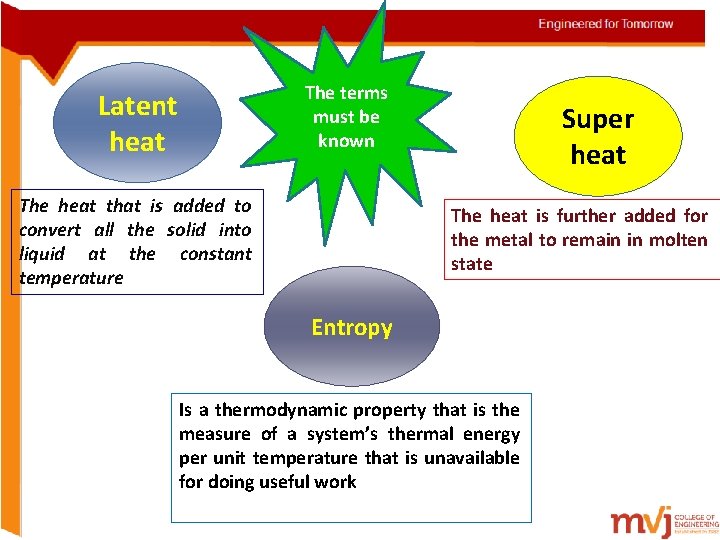
The terms must be known Latent heat The heat that is added to convert all the solid into liquid at the constant temperature Super heat The heat is further added for the metal to remain in molten state Entropy Is a thermodynamic property that is the measure of a system’s thermal energy per unit temperature that is unavailable for doing useful work
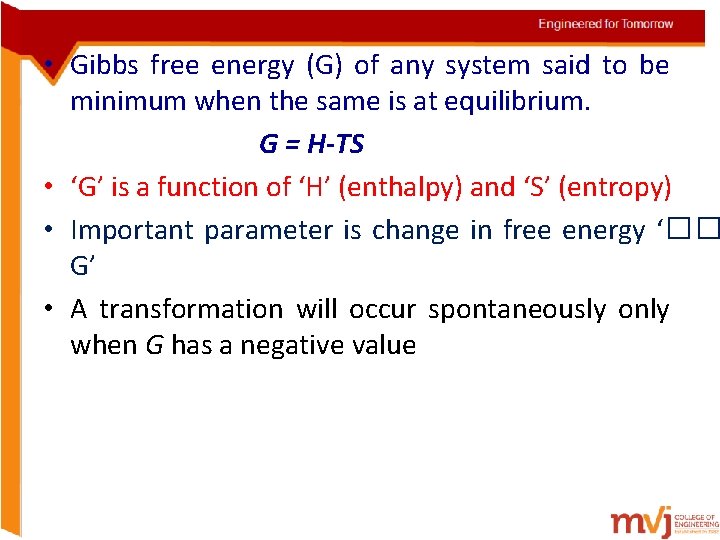
• Gibbs free energy (G) of any system said to be minimum when the same is at equilibrium. G = H-TS • ‘G’ is a function of ‘H’ (enthalpy) and ‘S’ (entropy) • Important parameter is change in free energy ‘�� G’ • A transformation will occur spontaneously only when G has a negative value

Ice melting in a warm room is a common example of increasing entropy
• A crystalline solid has lower internal energy and high degree of order, or lower entropy as compared to the liquid-phase i. e. , • Liquid has higher internal energy (equal to the heat of fusion) and higher entropy due to the more random structure
• Transformation from liquid metal to solid metal is accompanied by a shrinkage in the volume • This volume shrinkage takes place in three stages: 1. Liquid – Liquid 2. Liquid – Solid 3. Solid – Solid
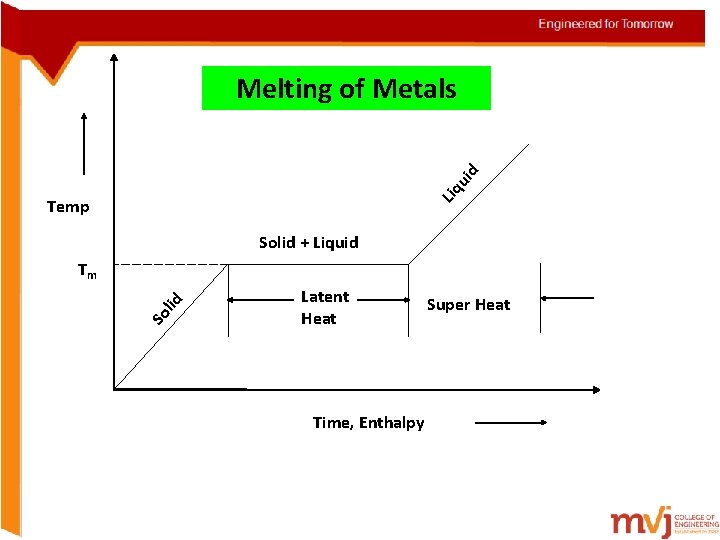
Liq ui d Melting of Metals Temp Solid + Liquid So l id Tm Latent Heat Time, Enthalpy Super Heat
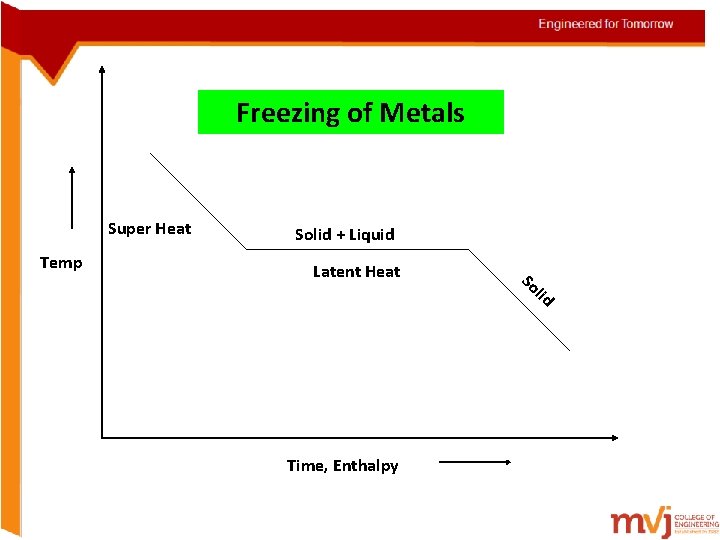
Freezing of Metals Super Heat Temp Solid + Liquid Latent Heat Time, Enthalpy So lid

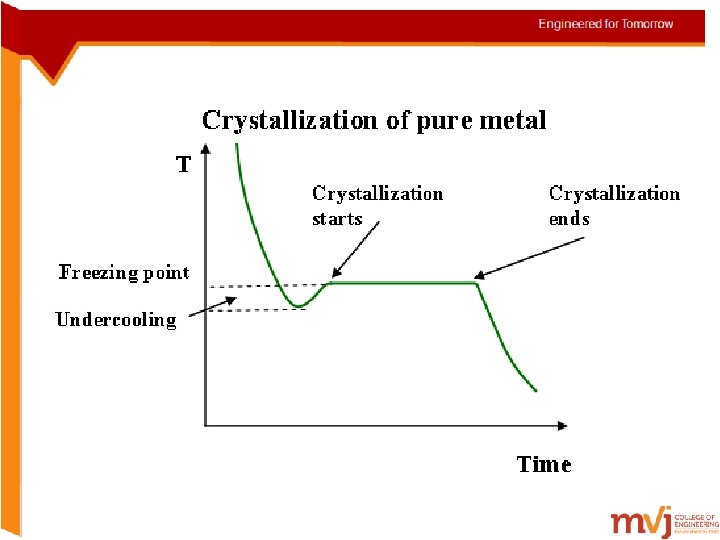
Undercooling (or) Supercooling in pure metals Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid • In alloys, commencement of solidification is easy since the foreign atoms act as source of nucleation • But pure metals experience difficulties in commencing solidification. (there are no foreign atoms to form nuclei) • In such cases the metal cools below its freezing temperature and actual solidification begins at the same point (shown in pic in the next slide)
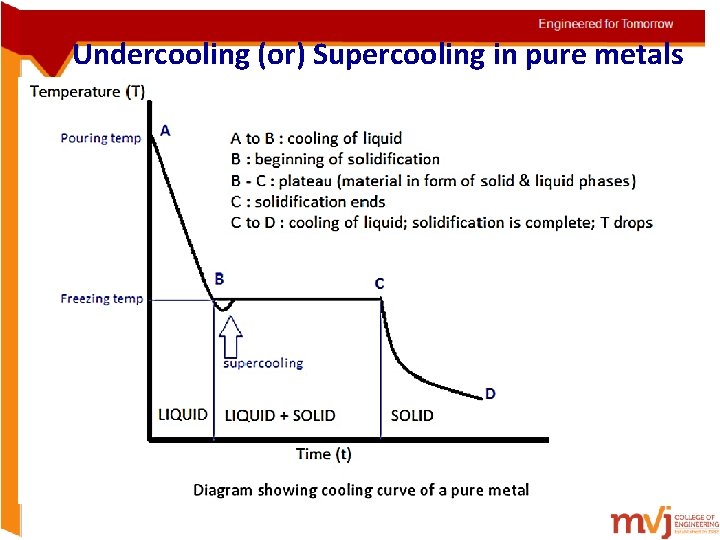
Undercooling (or) Supercooling in pure metals
Solidification of alloys • Solidification in alloys takes place in the same manner but with exceptions • They solidify over a range of temp rather than at a constant temp i. Begin solidification at one temp and end at another temp (Solid solution) ii. Begin and end solidification at a constant temp just like in pure metals (pure eutectics) iii. Begin solidification like a solid-solution and it like a eutectic
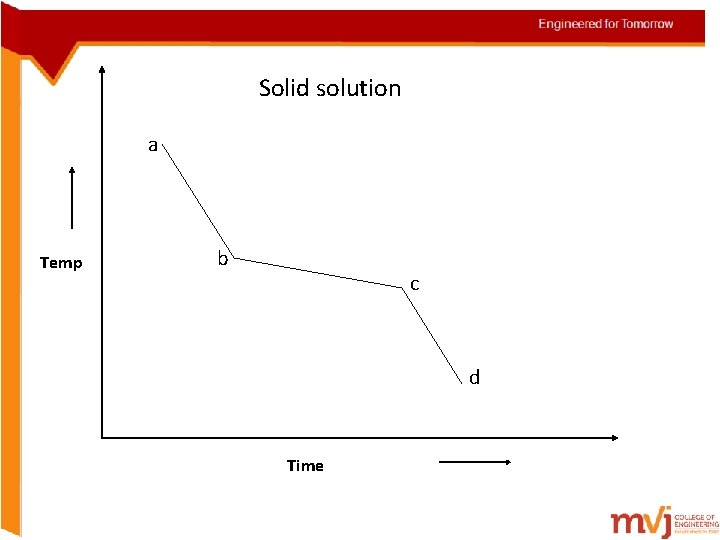
Solid solution a Temp b c d Time
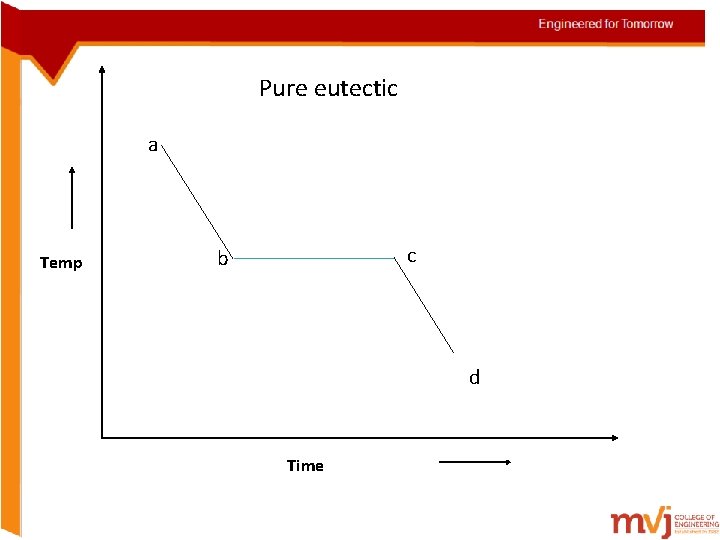
Pure eutectic a Temp c b d Time
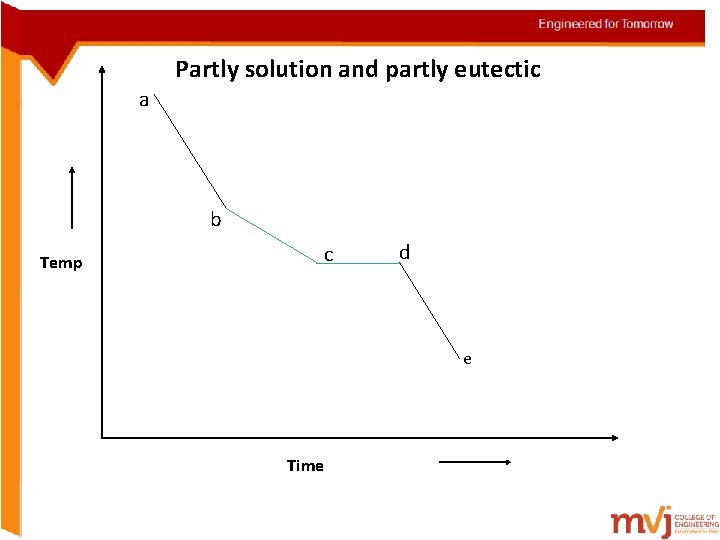
Partly solution and partly eutectic a b Temp c d e Time
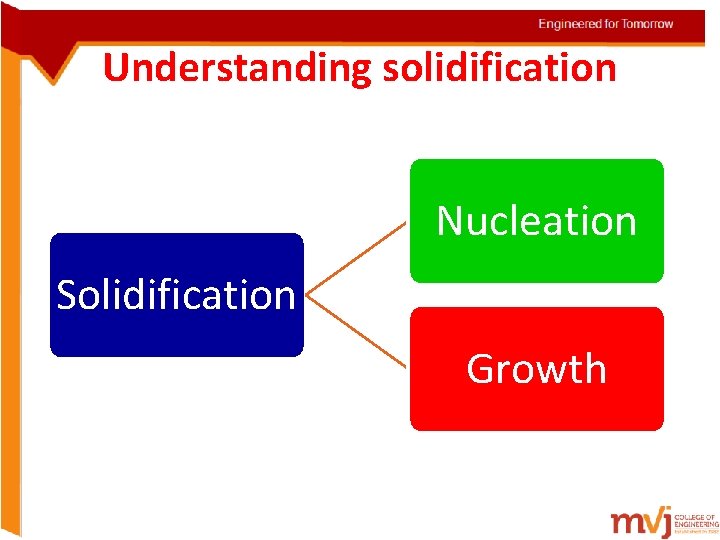
Understanding solidification Nucleation Solidification Growth
• The basic solidification process involves nucleation and growth • Nucleation involves the appearance of very small particles, or nuclei of the new phase (often consisting of only a few hundred atoms), which are capable of growing. • During the growth stage these nuclei increase in size, which results in the disappearance of some (or all) of the parent phase. • The transformation reaches completion if the growth of these new phase particles is allowed to proceed until the equilibrium fraction is attained
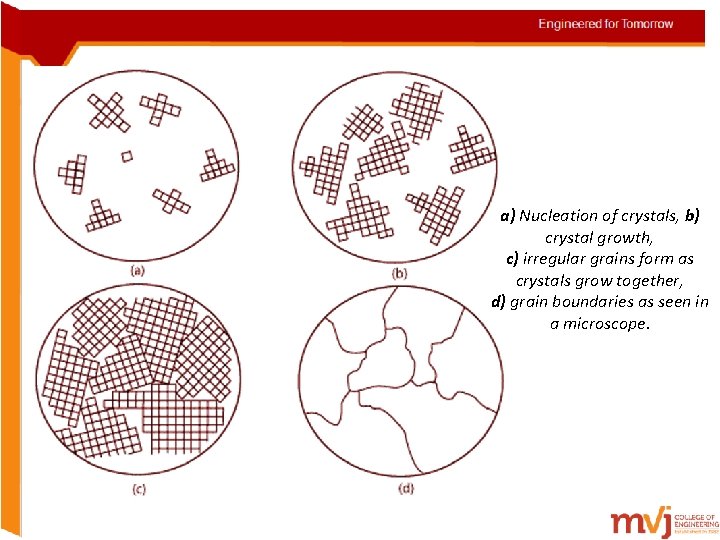
a) Nucleation of crystals, b) crystal growth, c) irregular grains form as crystals grow together, d) grain boundaries as seen in a microscope.
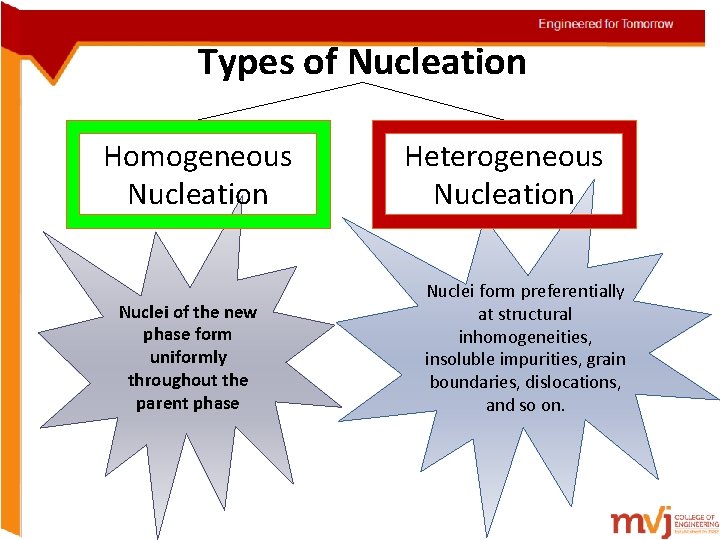
Types of Nucleation Homogeneous Nucleation Nuclei of the new phase form uniformly throughout the parent phase Heterogeneous Nucleation Nuclei form preferentially at structural inhomogeneities, insoluble impurities, grain boundaries, dislocations, and so on.
Homogeneous nucleation • Prominent is pure metals • Nuclei of the solid phase form in the interior of the liquid as atoms cluster together
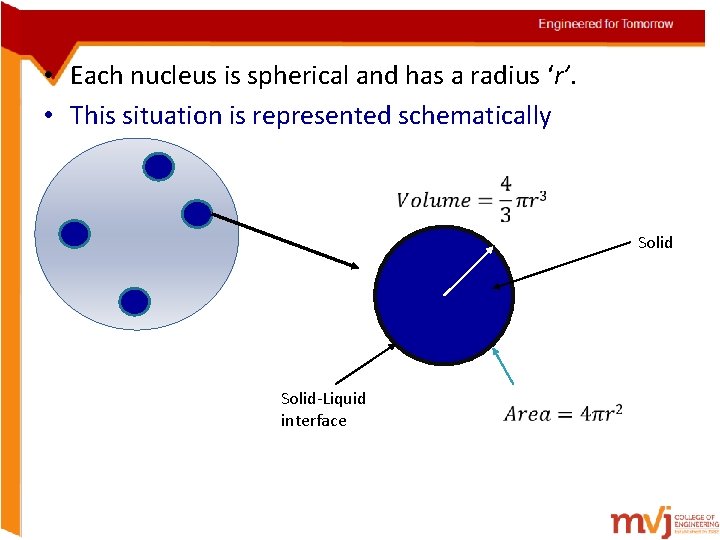
• Each nucleus is spherical and has a radius ‘r’. • This situation is represented schematically Solid-Liquid interface
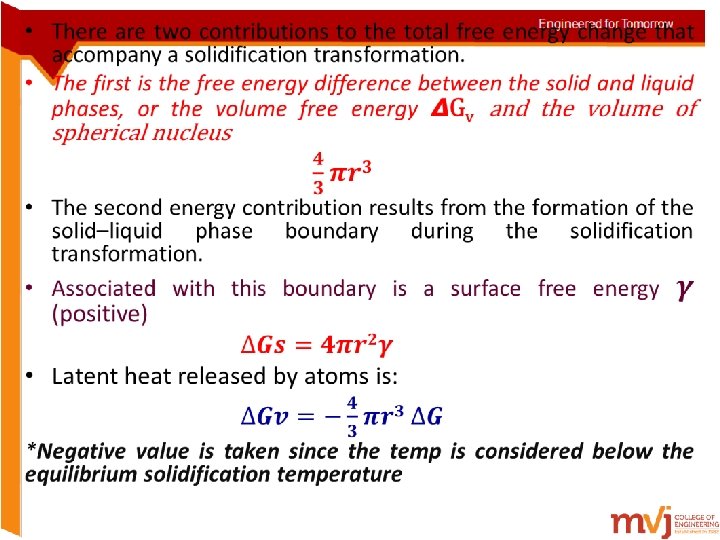
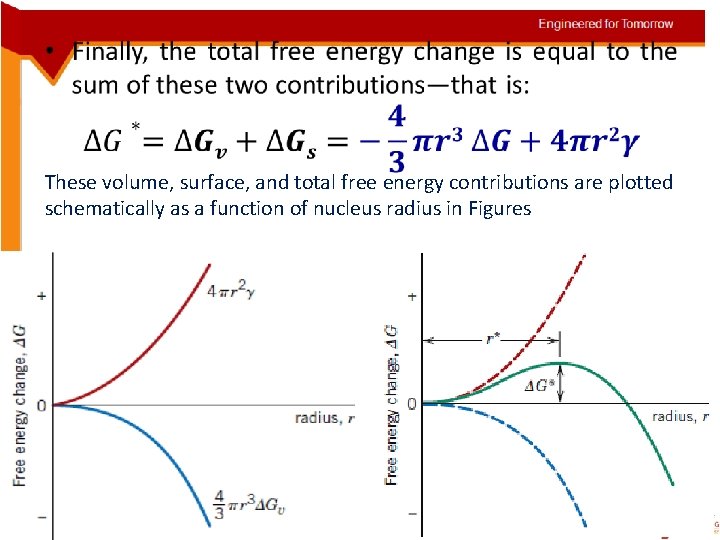
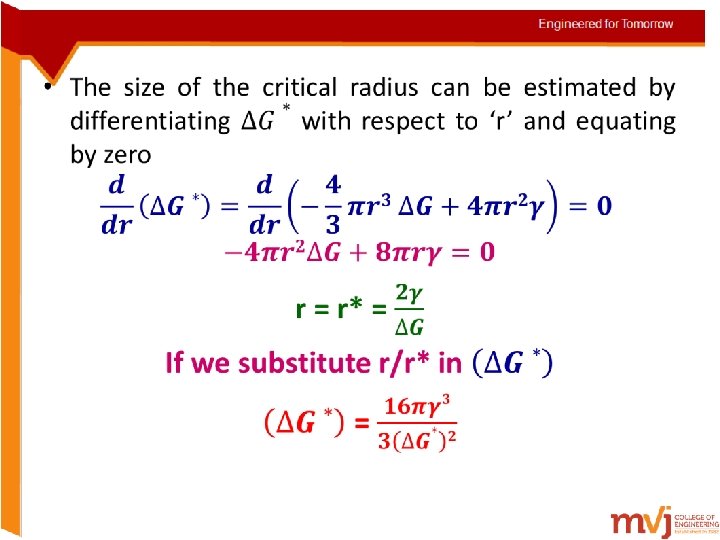
• These volume, surface, and total free energy contributions are plotted schematically as a function of nucleus radius in Figures
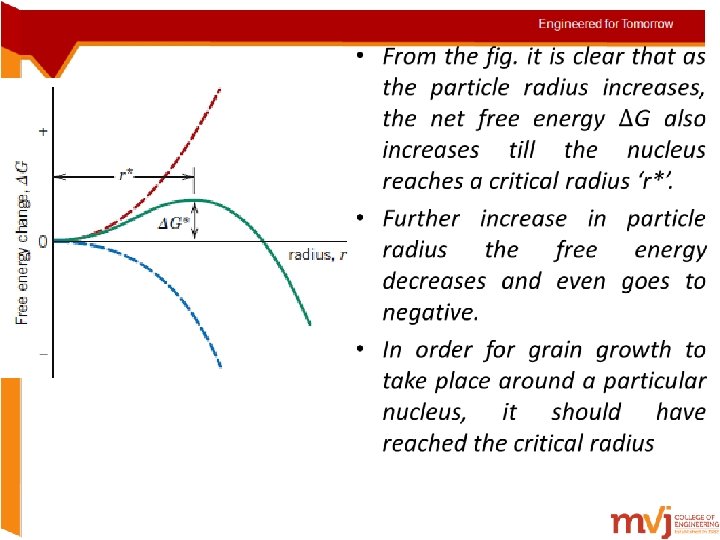
Heterogeneous nucleation • It is easier for nucleation to occur at surfaces and interfaces than at other sites. • Nucleation occurs with the help of impurities or chemical inhomogeneities. • Impurities can be insoluble like sand particles or alloying elements • Nuclei are formed on the surfaces of the above possible surfaces often called the ‘substrate’

Nucleation of carbon dioxide bubbles around a finger
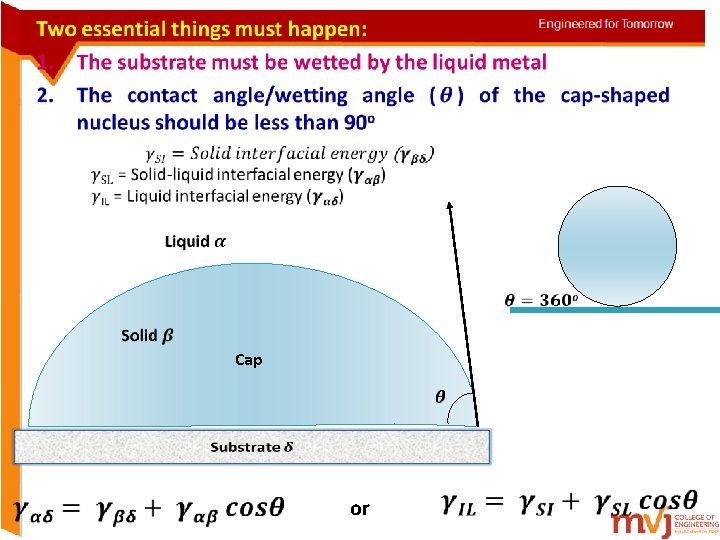
• Cap or
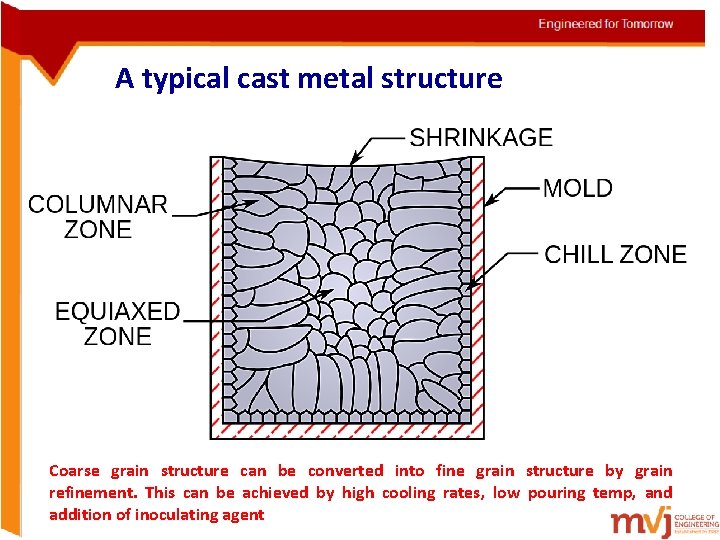
A typical cast metal structure Coarse grain structure can be converted into fine grain structure by grain refinement. This can be achieved by high cooling rates, low pouring temp, and addition of inoculating agent
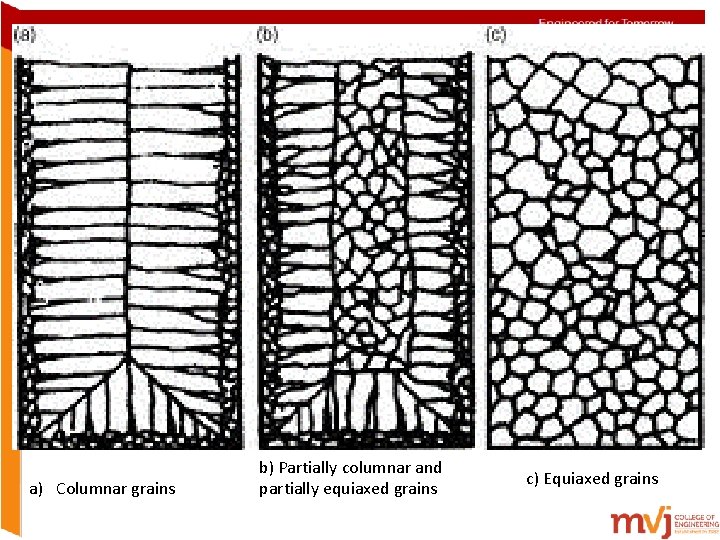
a) Columnar grains b) Partially columnar and partially equiaxed grains c) Equiaxed grains
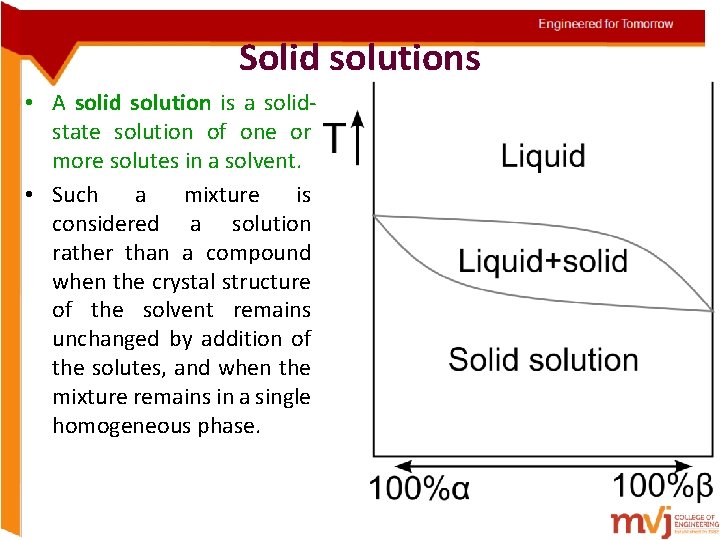
Solid solutions • A solid solution is a solidstate solution of one or more solutes in a solvent. • Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase.
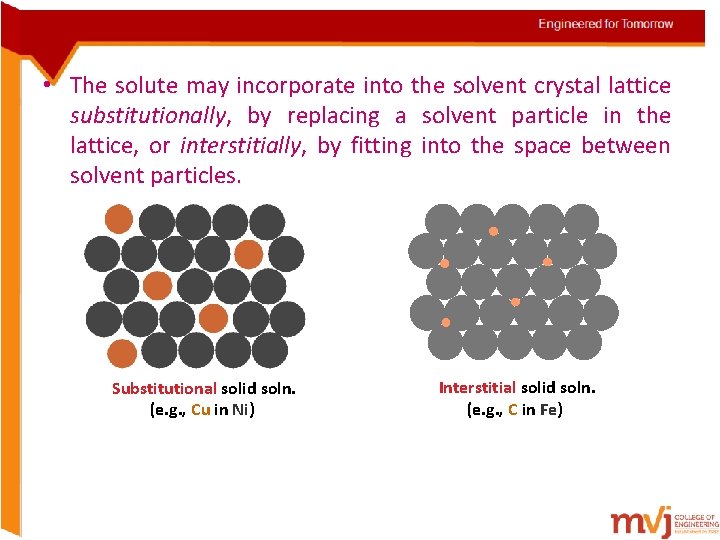
• The solute may incorporate into the solvent crystal lattice substitutionally, by replacing a solvent particle in the lattice, or interstitially, by fitting into the space between solvent particles. Substitutional solid soln. (e. g. , Cu in Ni) Interstitial solid soln. (e. g. , C in Fe)
Hari Prasad
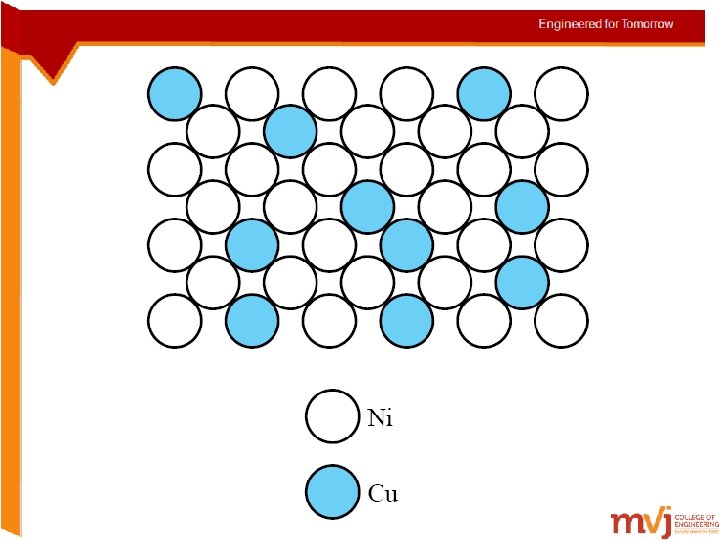
Conditions for substitutional solid solution (S. S. ) • W. Hume – Rothery rule – 1. r (atomic radius) < 15% – 2. Proximity in periodic table • i. e. , similar electronegativities – 3. Same crystal structure for pure metals – 4. Valency • Other factors being equal, a metal will have more of a tendency to dissolve another metal of higher valency than one of a lower valency. Hari Prasad
Intermediate phases • If a solid solution neither forms a substitutional type nor interstitial type, it certainly forms an intermediate compound. • And the compound is said to be “intermediate phase” or “intermediate compound” or “intermetallic” if it has metal in it.
Common intermediate compounds • Intermetallic or valency compounds • Interstitial compounds • Electron compounds
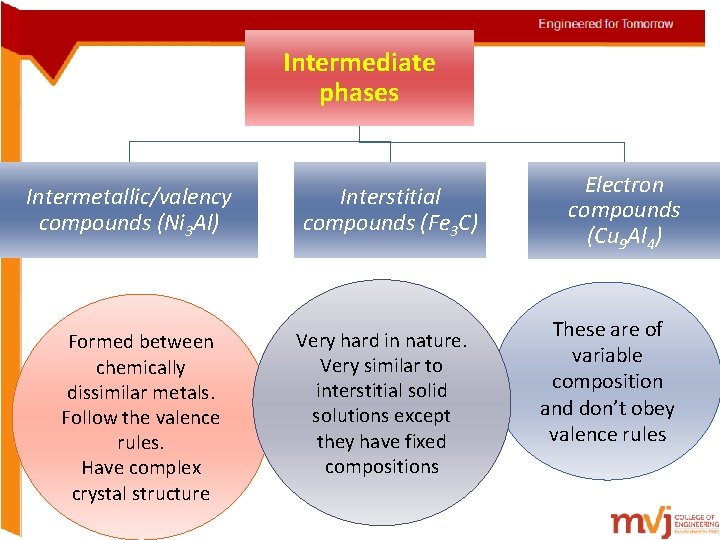
Intermediate phases Intermetallic/valency compounds (Ni 3 Al) Formed between chemically dissimilar metals. Follow the valence rules. Have complex crystal structure Interstitial compounds (Fe 3 C) Very hard in nature. Very similar to interstitial solid solutions except they have fixed compositions Electron compounds (Cu 9 Al 4) These are of variable composition and don’t obey valence rules
- Slides: 39