Unit 4 Solutions Chemists have Solutions Definitions Solution








- Slides: 12

Unit 4: Solutions (Chemists have Solutions!)

Definitions • Solution: Homogenous mixture of two or more substances in a single phase • Solvent: is the component of a solution that exists in the greatest quantity. • Solute: is the component of a solution that exists in the smaller quantity. • Saturated Solution: A solvent is said to be saturated with a solute, when no more solute will dissolve into it. • Unsaturated Solution: is one in which more solute can be dissolved

Definitions • Soluble: a solute is soluble in a solvent if the solute and the solvent mix to form a homogenous mixture. • Insoluble: a solute is said to be insoluble in a solvent if less than 0. 1 moles of the solute will dissolve in a liter of solution • Solubility: is the maximum amount of a given solute that dissolves in a given solvent at a given temperature. I. e. , when measuring the solubility of a system it must be for a saturated solution.

Units of Solubility • Solubility is the maximum concentration of a solute in a solvent for a given temperature. Units of solubility are: – Grams(solute)/liter of solution – Moles(solute)/liter of solution (M, molarity)

Determining Solubility • Must prepare a saturated solution – Use an xs amount of solute (goal is to have some un-dissolved solute separate from the solution) – Mix thoroughly and let sit over night at a constant temperature • Pipette a known amount of the saturated solution (25. 00 ml) into a pre-weighed weigh boat • Evaporate solution to dryness (remove solvent) • Determine the weight of the solute • Calculate solubility in grams/liter (convert to mol/L if required

Properties of Solutions • Conductivity: is a measure of how well a solution conducts electricity. ( for a solution to conduct electricity, it must contained charged particles, I. e. , IONS) • p. H: the p. H of a solution is a measure of how much H+ ions are in solution, the lower the p. H the more H+ ions there are present , the Higher the p. H the less H+ ions there are. Neutral water has a p. H of 7. 0 ( at 25 C. )

Dissolving Vs. Dissociating • Dissociating is when an Ionic compound breaks down into its ions in solution • Dissolving is when a covalent compound is absorbed by the solution, however it keeps its same chemical composition.

The Theory of Solubility • Polar solvents will dissociate ionic compounds and dissolve polar covalent compounds • Non-polar solvents will dissolve only non-polar covalent compounds.

What is Polarity? • A compound is said to be polar if it processes a net permanent dipole moment that do not cancel each other out. • Permanent dipole moments occur in covalent molecules where bonds exists between atoms with different electron-negativity. • A Net permanent dipole occurs when all the dipoles in a molecule do not cancel each other out.

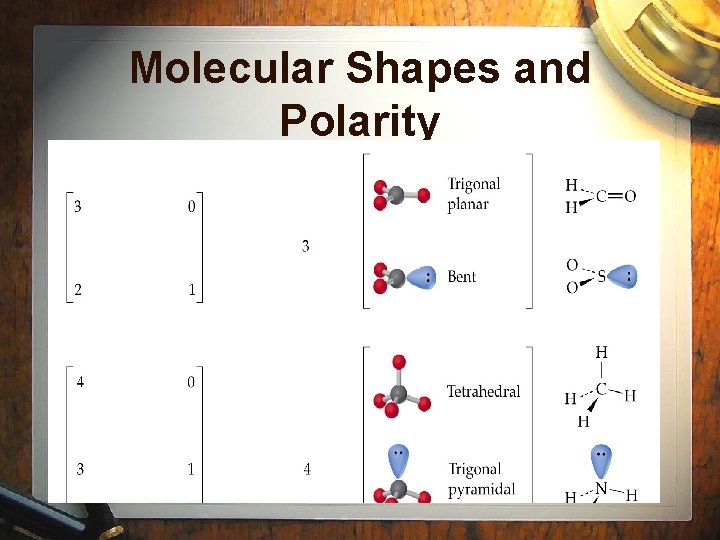
Molecular Shapes and Polarity

Importance of Polarity • Polarity effects the solubility of a solute in a given solution • Polarity effects the phase a pure compound will likely be in • Polarity effects the shape of large molecules such as proteins

Intermolecular Forces Vs. Intramolecular Forces • Intramolecular forces: are the forces internal to the molecule. They are responsible for holding the atoms in the molecule together. • Intermolecular Forces: are the forces between molecules – Dipole/Dipole interactions (polarity) – Hydrogen bonding – London forces