Unit 4 Solutions and Kinetics Rate Laws Concentration

![Rate Law Example #1 Reaction: A + B C Trial [A] [B] Rate (M/sec) Rate Law Example #1 Reaction: A + B C Trial [A] [B] Rate (M/sec)](https://slidetodoc.com/presentation_image_h2/5724a913abc718c835c8d2bf648973e9/image-5.jpg)
![Rate Law Practice #1 Reaction: A B + C Trial [A] Rate (M/sec) 1 Rate Law Practice #1 Reaction: A B + C Trial [A] Rate (M/sec) 1](https://slidetodoc.com/presentation_image_h2/5724a913abc718c835c8d2bf648973e9/image-6.jpg)
![Rate Law Practice #2 Reaction: A + B C Trial [A] [B] Rate (M/sec) Rate Law Practice #2 Reaction: A + B C Trial [A] [B] Rate (M/sec)](https://slidetodoc.com/presentation_image_h2/5724a913abc718c835c8d2bf648973e9/image-7.jpg)
![Rate Law Practice #3 Reaction: A + B + C D Trial [A] [B] Rate Law Practice #3 Reaction: A + B + C D Trial [A] [B]](https://slidetodoc.com/presentation_image_h2/5724a913abc718c835c8d2bf648973e9/image-8.jpg)
- Slides: 8

Unit 4: Solutions and Kinetics Rate Laws

Concentration Effect on Reaction Rates l Increasing the concentration of a reactant usually increases the rate of reaction. l HOWEVER, increased concentration might actually have little effect on the rate of reaction. l How can we tell?

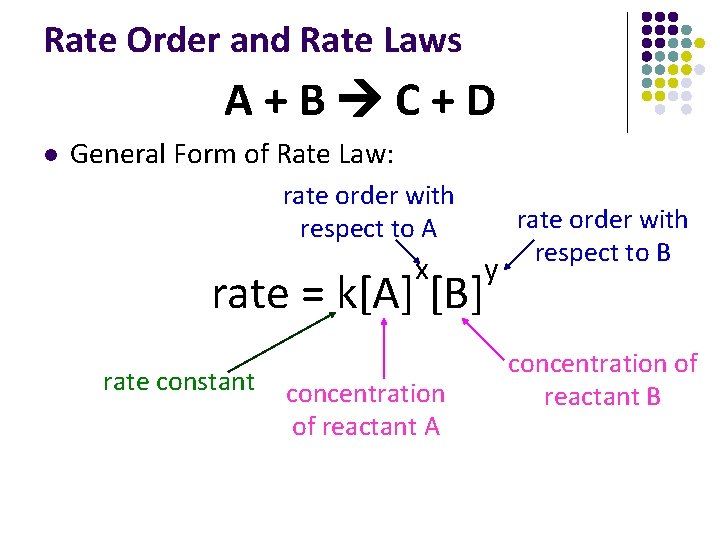
Rate Order and Rate Laws A+B C+D l General Form of Rate Law: rate order with respect to A x rate = k[A] [B] rate constant concentration of reactant A y rate order with respect to B concentration of reactant B

Rate Order and Rate Laws l Rate orders are found experimentally l l Change the concentration of one reactant at a time to see how the rates are affected Rate units: M/s (change in Molarity per second)
![Rate Law Example 1 Reaction A B C Trial A B Rate Msec Rate Law Example #1 Reaction: A + B C Trial [A] [B] Rate (M/sec)](https://slidetodoc.com/presentation_image_h2/5724a913abc718c835c8d2bf648973e9/image-5.jpg)
Rate Law Example #1 Reaction: A + B C Trial [A] [B] Rate (M/sec) 1 1. 0 2. 0 0. 50 2 2. 0 1. 00 3 2. 0 6. 0 3. 00 1. What happens to the rate when [A] doubles? 2. What is the rate order of reactant A? 3. What happens to the rate when [B] triples? 4. What is the rate order of reactant B? 5. What is the rate law for this reaction?
![Rate Law Practice 1 Reaction A B C Trial A Rate Msec 1 Rate Law Practice #1 Reaction: A B + C Trial [A] Rate (M/sec) 1](https://slidetodoc.com/presentation_image_h2/5724a913abc718c835c8d2bf648973e9/image-6.jpg)
Rate Law Practice #1 Reaction: A B + C Trial [A] Rate (M/sec) 1 2. 5 1. 00 2 5. 0 4. 00 3 7. 5 16. 00 1. What happens to the rate when [A] doubles? 2. What is the rate order (the exponent) of reactant A? 3. What is the rate law for this reaction?
![Rate Law Practice 2 Reaction A B C Trial A B Rate Msec Rate Law Practice #2 Reaction: A + B C Trial [A] [B] Rate (M/sec)](https://slidetodoc.com/presentation_image_h2/5724a913abc718c835c8d2bf648973e9/image-7.jpg)
Rate Law Practice #2 Reaction: A + B C Trial [A] [B] Rate (M/sec) 1 2. 0 4. 0 3. 0 2 6. 0 2. 0 1. 5 3 6. 0 4. 0 3. 0 1. What happens to the rate when [A] triples? 2. What is the rate order of reactant A? 3. What happens to the rate when [B] doubles? 4. What is the rate order of reactant B? 5. What is the rate law for this reaction?
![Rate Law Practice 3 Reaction A B C D Trial A B Rate Law Practice #3 Reaction: A + B + C D Trial [A] [B]](https://slidetodoc.com/presentation_image_h2/5724a913abc718c835c8d2bf648973e9/image-8.jpg)
Rate Law Practice #3 Reaction: A + B + C D Trial [A] [B] [C] Rate (M/sec) 1 1. 0 4. 0 3. 0 1. 0 2 1. 0 4. 0 6. 0 1. 0 3 1. 0 8. 0 3. 0 2. 0 4 2. 0 8. 0 3. 0 8. 0 1. What is the rate order for reactant A? 2. What is the rate order for reactant B? 3. What is the rate order for reactant C? 4. What is the rate law for this reaction?