UNIT 4 Nuclear Chemistry How Does an Unstable

UNIT 4 Nuclear Chemistry How Does an Unstable Nucleus Release Energy? How Can Radioisotopes Be Useful?

Nuclear Chemistry Radioactivity Types of Radiation Nuclear Equations
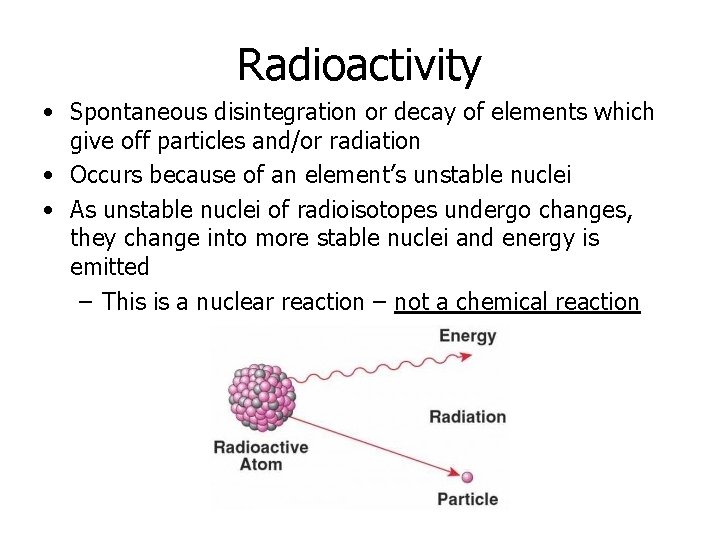
Radioactivity • Spontaneous disintegration or decay of elements which give off particles and/or radiation • Occurs because of an element’s unstable nuclei • As unstable nuclei of radioisotopes undergo changes, they change into more stable nuclei and energy is emitted – This is a nuclear reaction – not a chemical reaction

Radioactive Elements • All elements have at least one radioactive isotope. • Elements with atomic number > 83 are said to be naturally radioactive, meaning ALL isotopes of the element are unstable and therefore radioactive.

Henri Bequerel (1896) • Made accidental discovery • Uranium salts fogged photographic paper even without the sun • Called the rays emitted “U-Rays” and continued his studies without much success
Marie & Pierre Curie • Named the process that fogged plates radioactivity • Named the source of rays radiation • Found that Thorium acted the same as Uranium • Isolated Polonium- more radioactive than Thorium • Marie (after Pierre died) isolated Radium- more radioactive than Polonium
Unstable Nuclei • Radioisotopes have unstable nuclei due to the neutron to proton ratio. – When the n 0 : p+ ratio exceeds 1. 5: 1, the nucleus is unstable. – When the number of neutrons increases, this destabilizes the nucleus. • Band of stability – where the n 0 : p+ ratio is about 1: 1 – the most stable elements are found here – The farther away the ratio is from 1: 1, the more unstable the nuclei
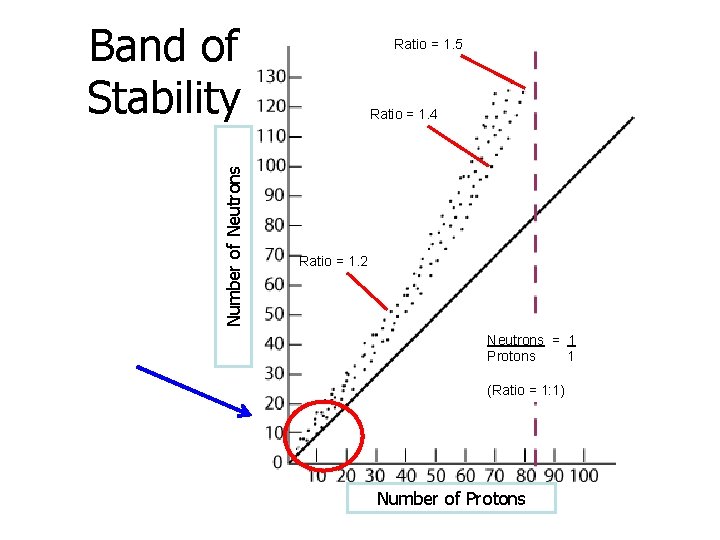
Number of Neutrons Band of Stability Ratio = 1. 5 Ratio = 1. 4 Ratio = 1. 2 Neutrons = 1 Protons 1 (Ratio = 1: 1) Number of Protons
Unstable Elements • The elements from #84 and above (85, 86, 87…) are all naturally radioactive because they are unstable. • So the element goes through a transmutation – A change from an unstable element to a stable isotope of a different element and emits a particle and energy • (Uranium Decay Series)

Radioactive Decay • When an unstable nucleus loses energy by emitting radiation. • Spontaneous process – Does not require any input of energy • Eventually, unstable radioisotopes are transformed into stable (non-radioactive) isotopes of a different element • Not all mass is conserved – Small amount of mass gets changed into energy – Called the “mass defect” E = mc 2
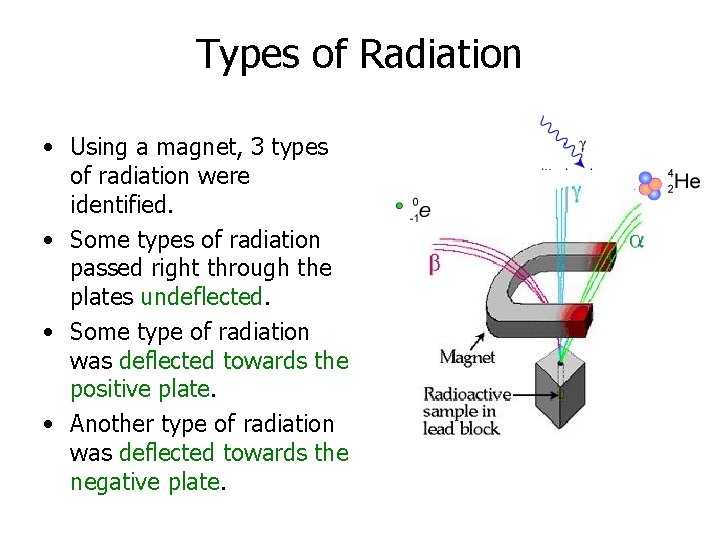
Types of Radiation • Using a magnet, 3 types of radiation were identified. • Some types of radiation passed right through the plates undeflected. • Some type of radiation was deflected towards the positive plate. • Another type of radiation was deflected towards the negative plate.
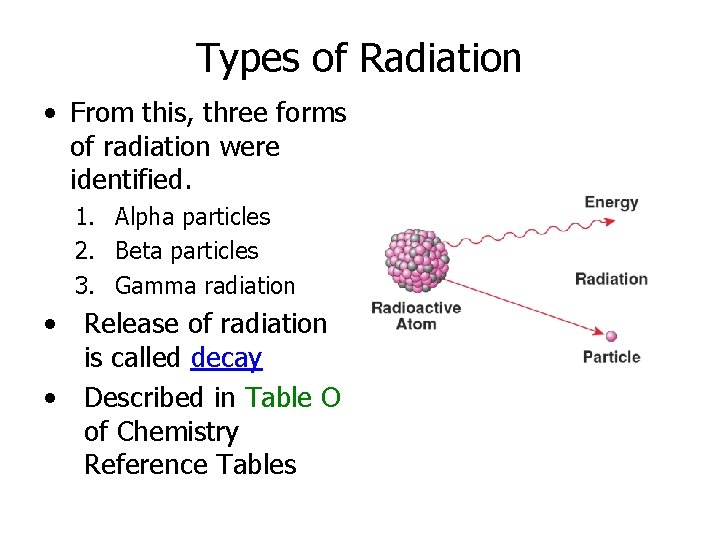
Types of Radiation • From this, three forms of radiation were identified. 1. Alpha particles 2. Beta particles 3. Gamma radiation • Release of radiation is called decay • Described in Table O of Chemistry Reference Tables
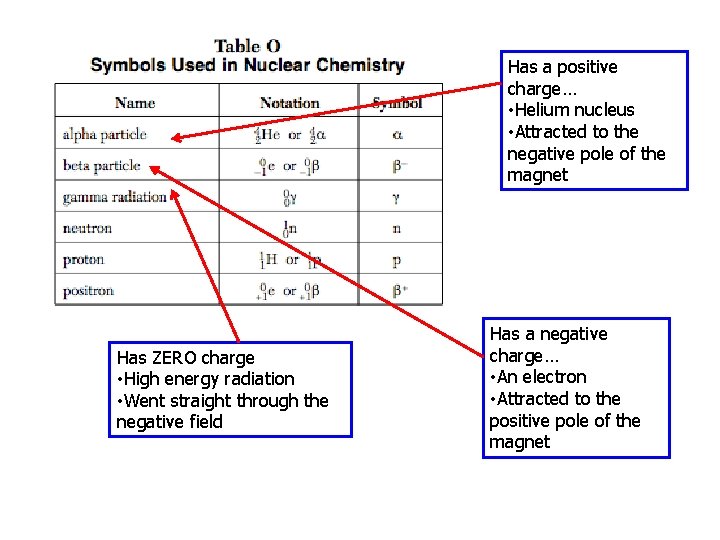
Has a positive charge… • Helium nucleus • Attracted to the negative pole of the magnet Has ZERO charge • High energy radiation • Went straight through the negative field Has a negative charge… • An electron • Attracted to the positive pole of the magnet
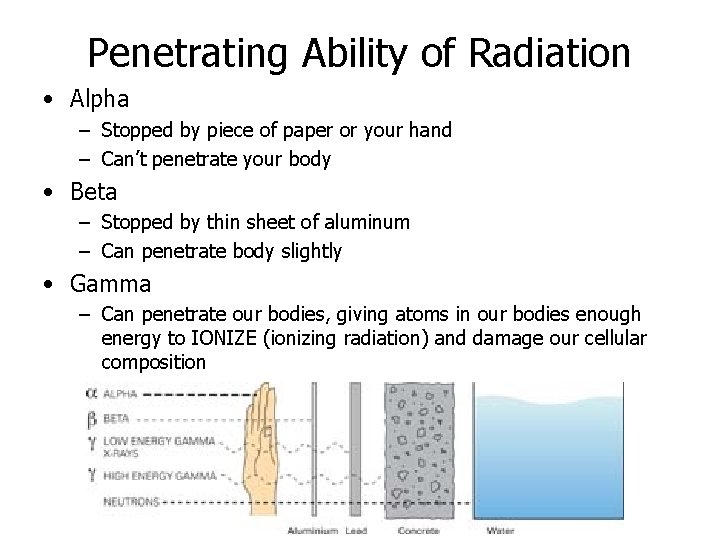
Penetrating Ability of Radiation • Alpha – Stopped by piece of paper or your hand – Can’t penetrate your body • Beta – Stopped by thin sheet of aluminum – Can penetrate body slightly • Gamma – Can penetrate our bodies, giving atoms in our bodies enough energy to IONIZE (ionizing radiation) and damage our cellular composition
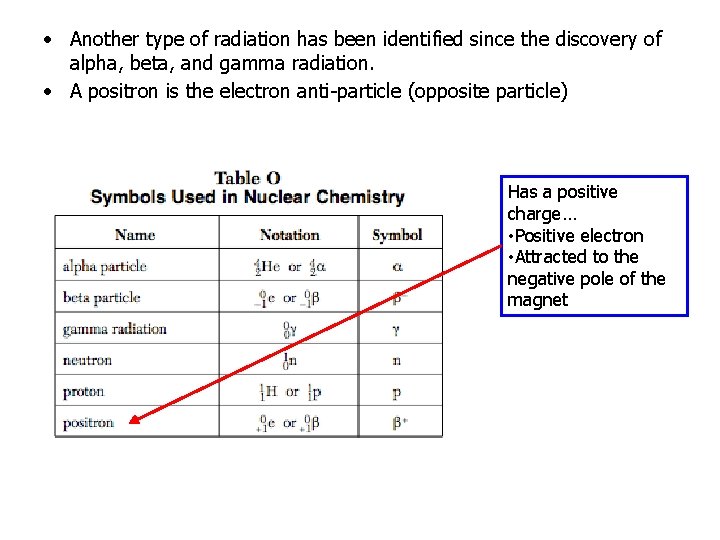
• Another type of radiation has been identified since the discovery of alpha, beta, and gamma radiation. • A positron is the electron anti-particle (opposite particle) Has a positive charge… • Positive electron • Attracted to the negative pole of the magnet

Radioactive elements change… • They undergo some form of radioactive decay where the element emits radiation to become a more stable nucleus (change in identity) • During this process, energy is released in the form of gamma radiation • This change in identity that occurs due to a change in the atomic number (number of protons in the nucleus) is called a transmutation
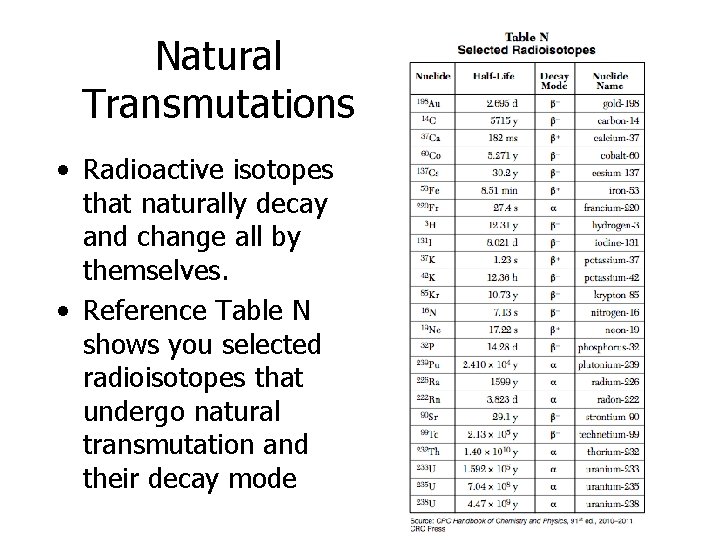
Natural Transmutations • Radioactive isotopes that naturally decay and change all by themselves. • Reference Table N shows you selected radioisotopes that undergo natural transmutation and their decay mode
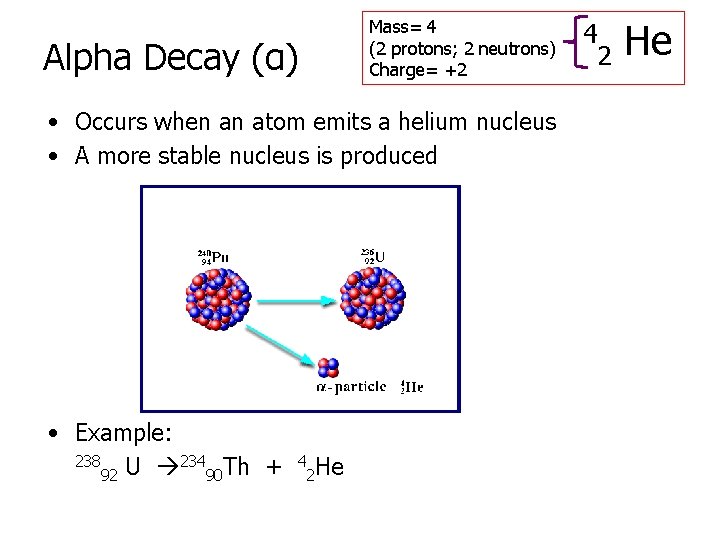
Mass= 4 (2 protons; 2 neutrons) Charge= +2 Alpha Decay (α) • Occurs when an atom emits a helium nucleus • A more stable nucleus is produced • Example: 238 234 Th + U 92 90 4 2 He 4 2 He

Practice alpha decay problems •
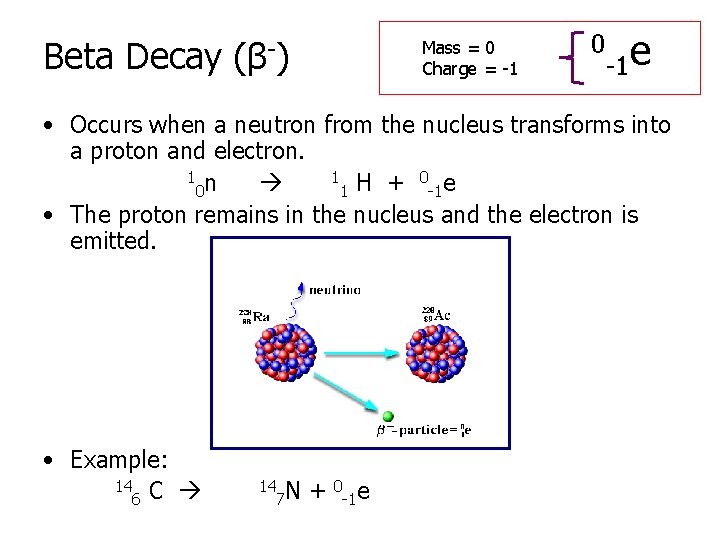
Beta Decay (β-) Mass = 0 Charge = -1 0 -1 e • Occurs when a neutron from the nucleus transforms into a proton and electron. 1 n 1 H + 0 e 0 1 -1 • The proton remains in the nucleus and the electron is emitted. • Example: 14 C 6 14 7 N + 0 -1 e
Practice beta decay problems •
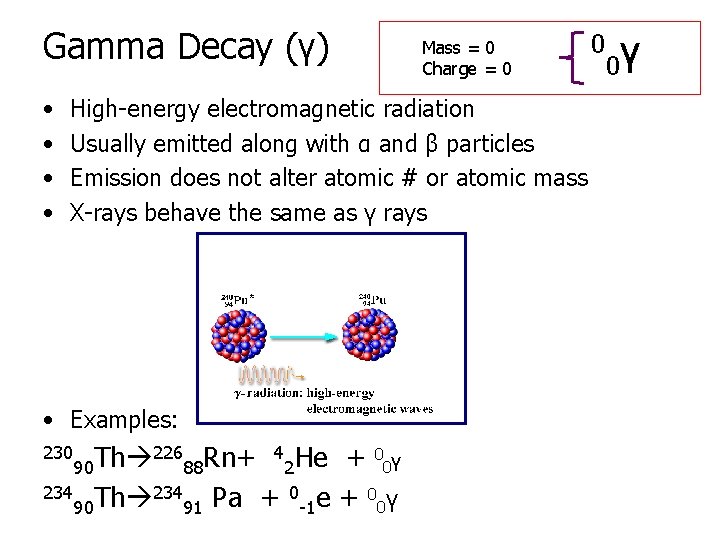
Gamma Decay (γ) • • Mass = 0 Charge = 0 High-energy electromagnetic radiation Usually emitted along with α and β particles Emission does not alter atomic # or atomic mass X-rays behave the same as γ rays • Examples: 226 Rn+ 4 He + 0 γ Th 0 90 88 2 234 Th 234 0 e + 0 γ Pa + 0 90 91 -1 230 0 0γ
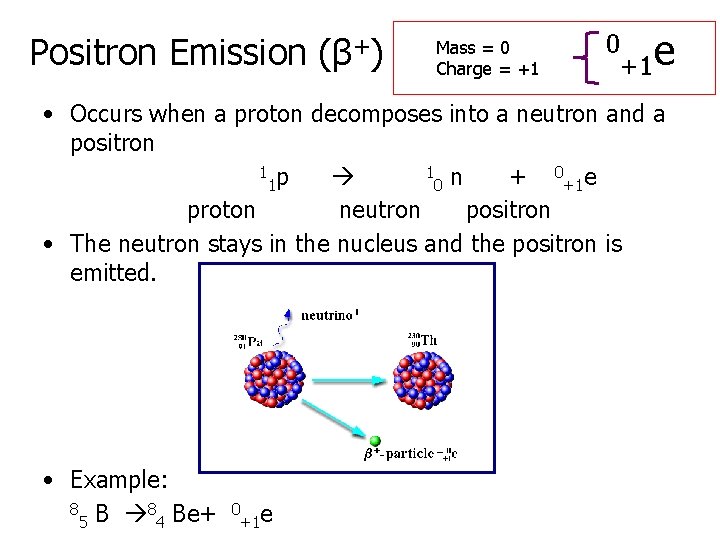
Positron Emission (β+) Mass = 0 Charge = +1 0 +1 e • Occurs when a proton decomposes into a neutron and a positron 1 p 1 n + 0+1 e 1 0 proton neutron positron • The neutron stays in the nucleus and the positron is emitted. • Example: 8 Be+ 5 4 0 +1 e
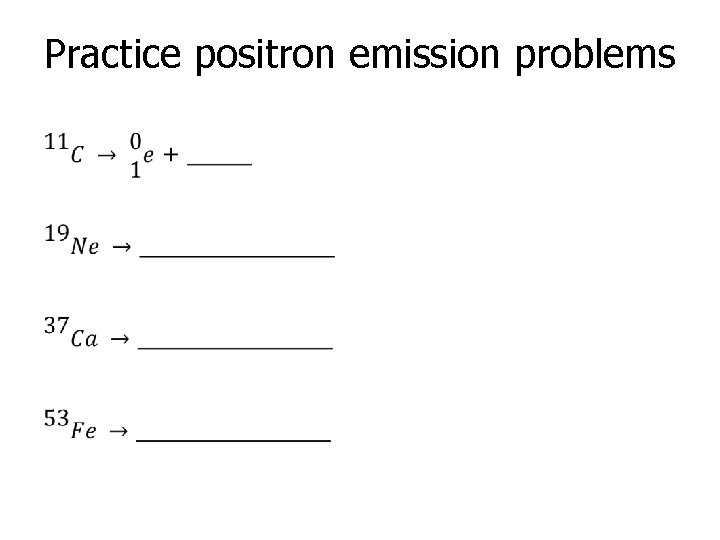
Practice positron emission problems •
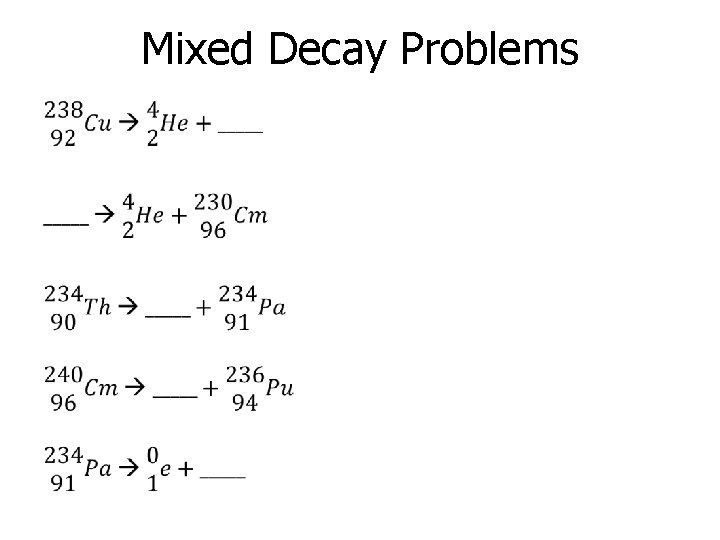
Mixed Decay Problems •
Nuclear Chemistry Half-Life
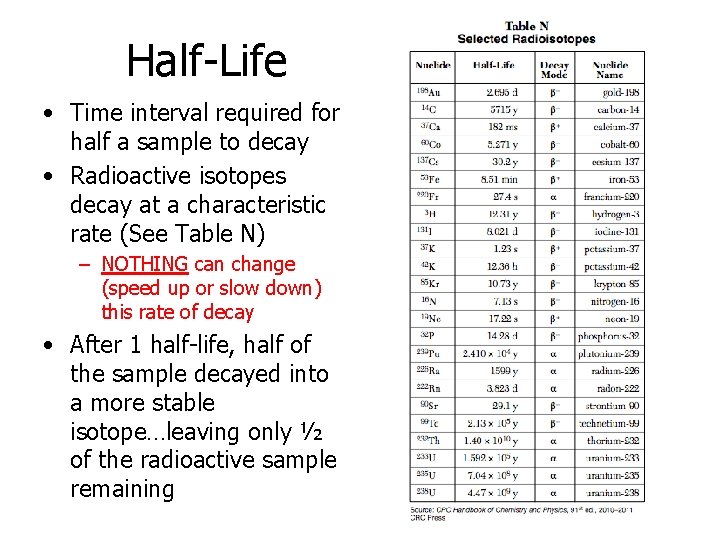
Half-Life • Time interval required for half a sample to decay • Radioactive isotopes decay at a characteristic rate (See Table N) – NOTHING can change (speed up or slow down) this rate of decay • After 1 half-life, half of the sample decayed into a more stable isotope…leaving only ½ of the radioactive sample remaining
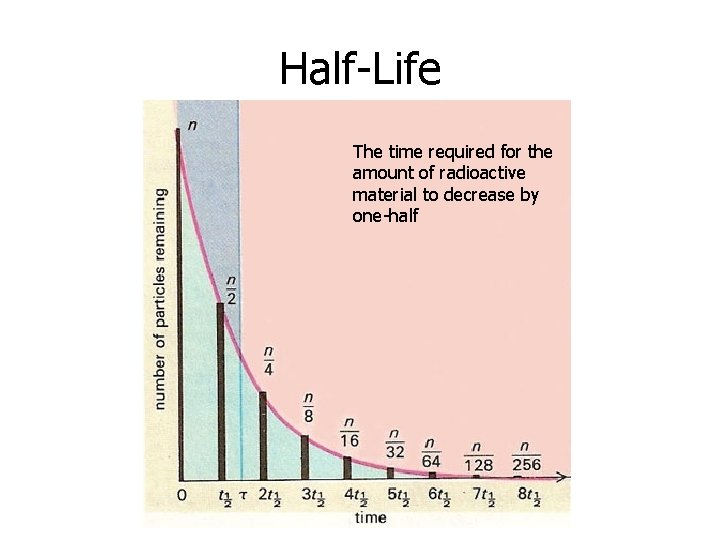
Half-Life The time required for the amount of radioactive material to decrease by one-half
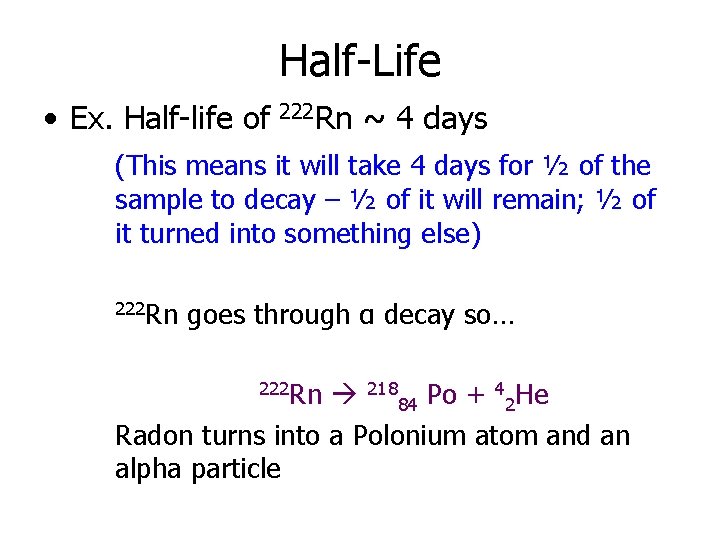
Half-Life • Ex. Half-life of 222 Rn ~ 4 days (This means it will take 4 days for ½ of the sample to decay – ½ of it will remain; ½ of it turned into something else) 222 Rn goes through α decay so… 222 Rn 21884 Po + 42 He Radon turns into a Polonium atom and an alpha particle
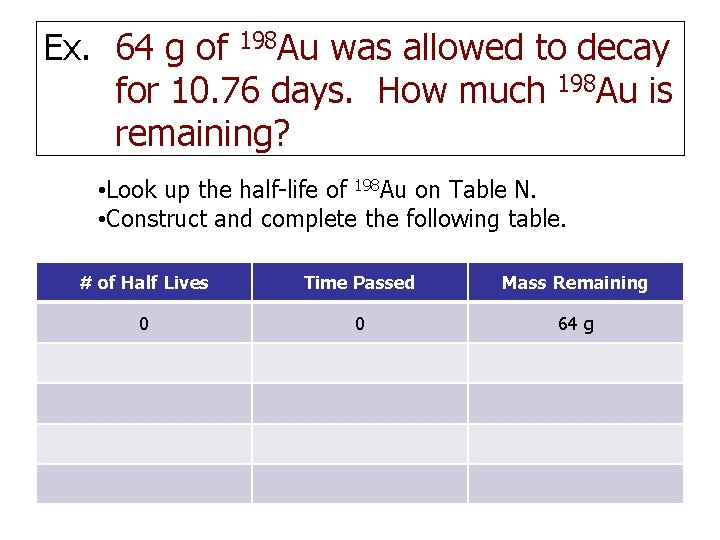
Ex. 64 g of 198 Au was allowed to decay for 10. 76 days. How much 198 Au is remaining? • Look up the half-life of 198 Au on Table N. • Construct and complete the following table. # of Half Lives Time Passed Mass Remaining 0 0 64 g
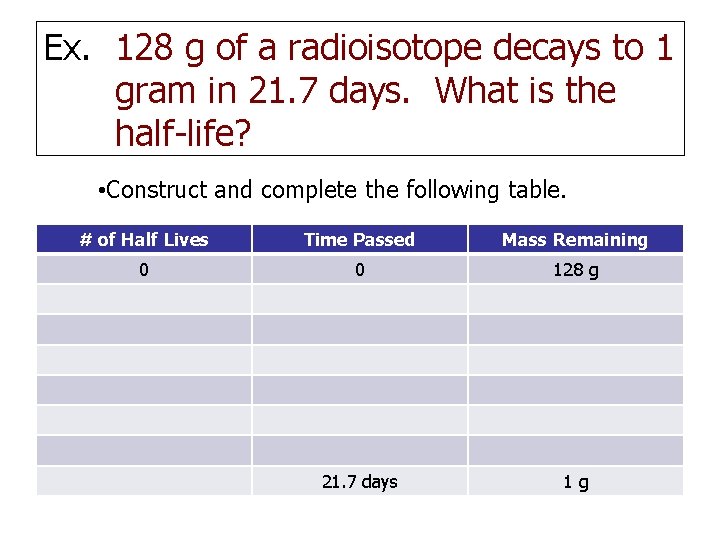
Ex. 128 g of a radioisotope decays to 1 gram in 21. 7 days. What is the half-life? • Construct and complete the following table. # of Half Lives Time Passed Mass Remaining 0 0 128 g 21. 7 days 1 g
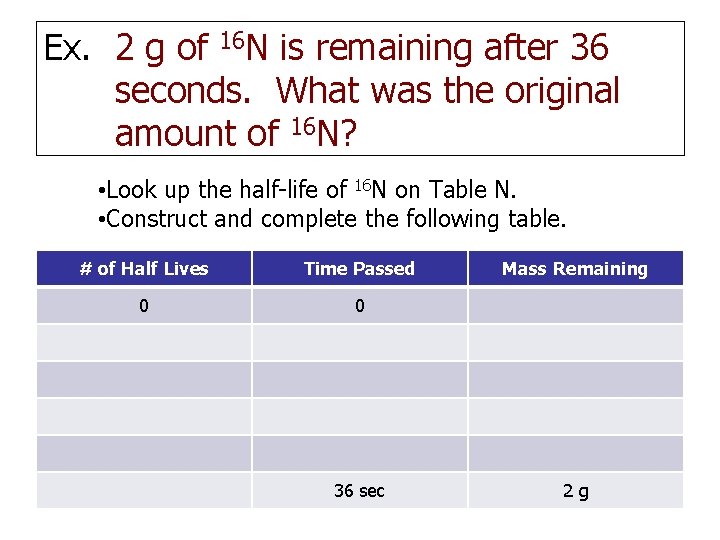
Ex. 2 g of 16 N is remaining after 36 seconds. What was the original amount of 16 N? • Look up the half-life of 16 N on Table N. • Construct and complete the following table. # of Half Lives Time Passed 0 0 36 sec Mass Remaining 2 g
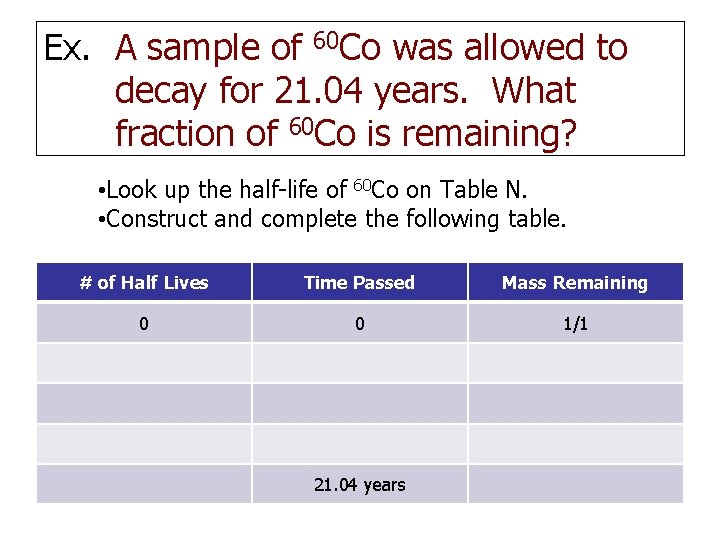
Ex. A sample of 60 Co was allowed to decay for 21. 04 years. What fraction of 60 Co is remaining? • Look up the half-life of 60 Co on Table N. • Construct and complete the following table. # of Half Lives Time Passed Mass Remaining 0 0 1/1 21. 04 years
Nuclear Chemistry Transmutations Fission & Fusion
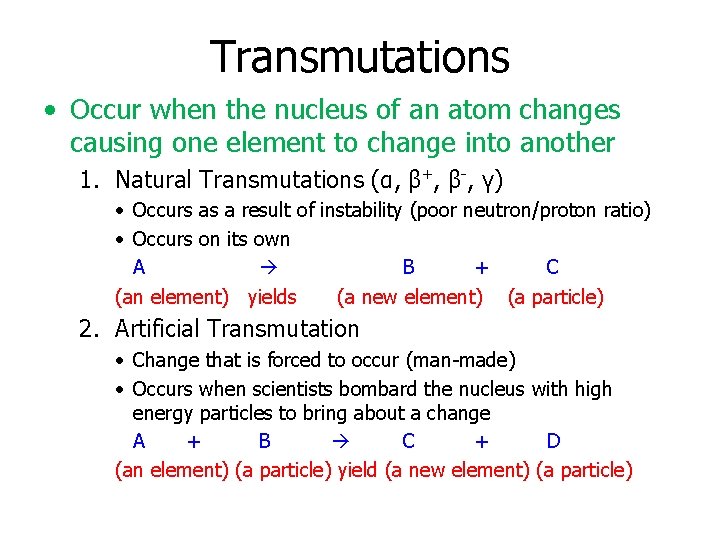
Transmutations • Occur when the nucleus of an atom changes causing one element to change into another 1. Natural Transmutations (α, β+, β-, γ) • Occurs as a result of instability (poor neutron/proton ratio) • Occurs on its own A B + C (an element) yields (a new element) (a particle) 2. Artificial Transmutation • Change that is forced to occur (man-made) • Occurs when scientists bombard the nucleus with high energy particles to bring about a change A + B C + D (an element) (a particle) yield (a new element) (a particle)
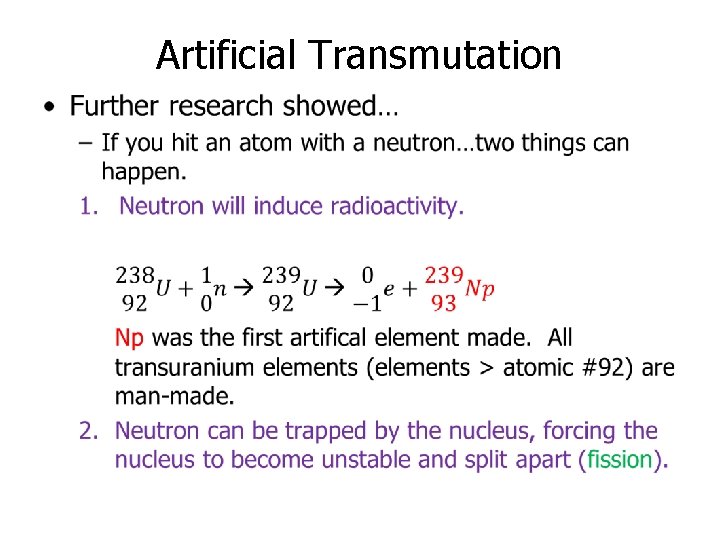
Artificial Transmutation •
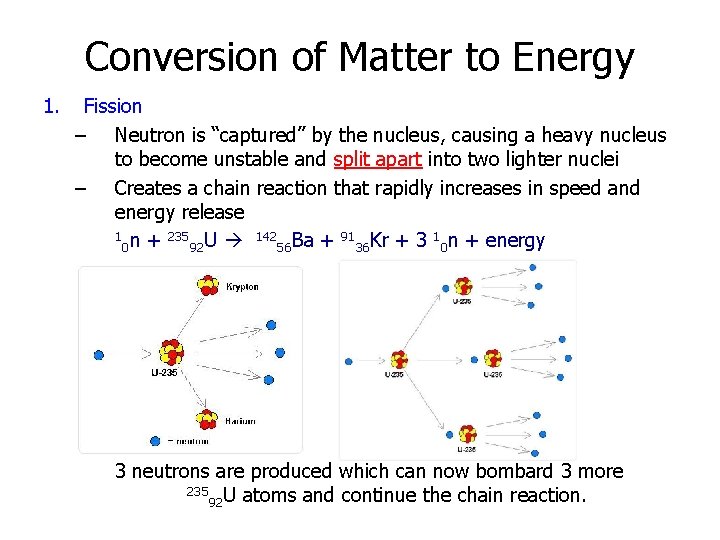
Conversion of Matter to Energy 1. Fission – Neutron is “captured” by the nucleus, causing a heavy nucleus to become unstable and split apart into two lighter nuclei – Creates a chain reaction that rapidly increases in speed and energy release 1 n + 235 U 142 Ba + 91 Kr + 3 1 n + energy 0 92 56 36 0 3 neutrons are produced which can now bombard 3 more 235 U atoms and continue the chain reaction. 92

• If more than one neutron from each fission event , causes another fission event, the process rapidly escalates, and the heat build up causes an extremely violent explosion (nuclear bomb) • Manhattan Project – U. S. carried out an intense research effort to build a bomb in an effort to win WWII – 1945 - Hiroshima & Nagasaki

Types of Fission • Controlled fission reactions used to make electricity within nuclear reactors • Uncontrolled fission reactions used to make nuclear bombs

Nuclear Accidents 1. • Three Mile Island – March 29, 1979 Equipment failures and human error at the Three Mile Island nuclear reactor in Harrisburg, Pennsylvania resulted in loss of reactor coolant, overheating of the core, and damage to the fuel but probably no melting, and limited releases outside the plant of radioactive noble gases and iodine. Hydrogen gas was formed, primarily by a reaction between zirconium and water, which is not unexpected when core overheating occurs. There was never a danger of the bubble inside the reactor vessel exploding because there was no oxygen present inside the vessel.

2. Chernobyl – April 26, 1986 • Due to a flawed reactor design that was operated with inadequately trained personnel, an explosion and a fire in the graphite core of one of four reactors occurred at Chernobyl in the Soviet Union. This accident released 5% of the radioactive reactor core into the atmosphere and then spread over part of the Soviet Union and Europe. Thirty-one people died (2 immediately and 29 later from radiation poisoning).

3. Fukushima – March 12, 2011 • The magnitude 9. 0 earthquake cut off outside power to the Fukushima Daiichi nuclear plant. The resulting tsunami disabled emergency generators required to operate the reactors’ cooling systems. In the five days following the quake, hydrogen gas explosions blasted through three of the plant’s six reactors, and nuclear fuel rods partially melted down in three reactors. Radiation releases forced more than 80, 000 people to evacuate, food and water supplies were contaminated, and several plant workers died.
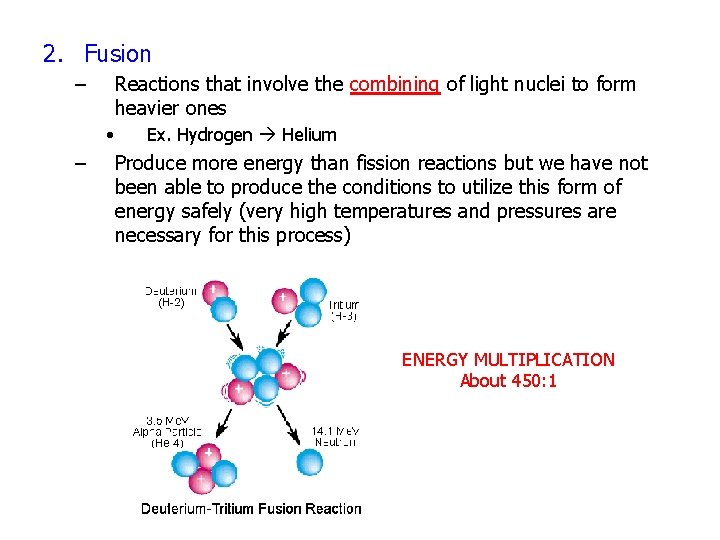
2. Fusion – Reactions that involve the combining of light nuclei to form heavier ones • – Ex. Hydrogen Helium Produce more energy than fission reactions but we have not been able to produce the conditions to utilize this form of energy safely (very high temperatures and pressures are necessary for this process) ENERGY MULTIPLICATION About 450: 1

• Fusion is the dominant reaction that power the Sun and other stars • On Earth, nuclear fusion was first reached in the explosion of the hydrogen bomb.

Nuclear Chemistry Benefits & Risks of Radioisotopes

Radioisotopes • Not all radiation is harmful!!! • Although radioisotopes release potentially harmful radiation, they have many practical applications in: Industry Medicine Research

Dating Fossils • Carbon-14 – Used to date previously living material – C-14 is found in all living material and naturally decays into C-12 after the organism dies – Ratio of C-14/C-12 tells age – Half-life of C-14 is 5730 years

Dating Rocks and Minerals •
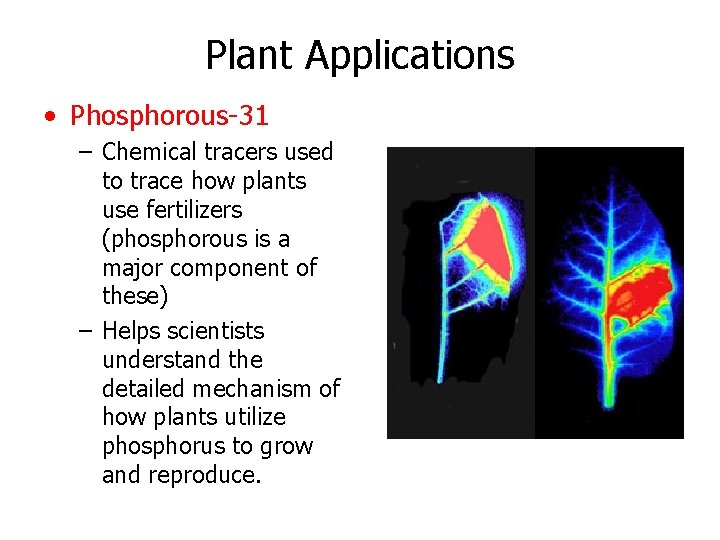
Plant Applications • Phosphorous-31 – Chemical tracers used to trace how plants use fertilizers (phosphorous is a major component of these) – Helps scientists understand the detailed mechanism of how plants utilize phosphorus to grow and reproduce.
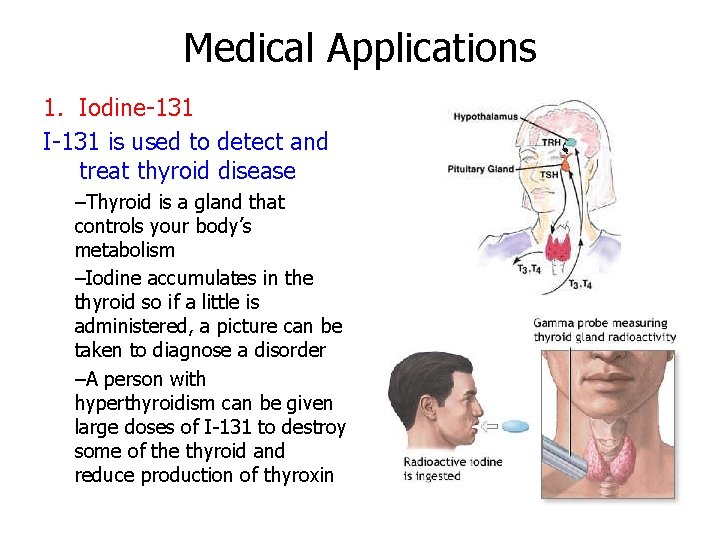
Medical Applications 1. Iodine-131 I-131 is used to detect and treat thyroid disease –Thyroid is a gland that controls your body’s metabolism –Iodine accumulates in the thyroid so if a little is administered, a picture can be taken to diagnose a disorder –A person with hyperthyroidism can be given large doses of I-131 to destroy some of the thyroid and reduce production of thyroxin
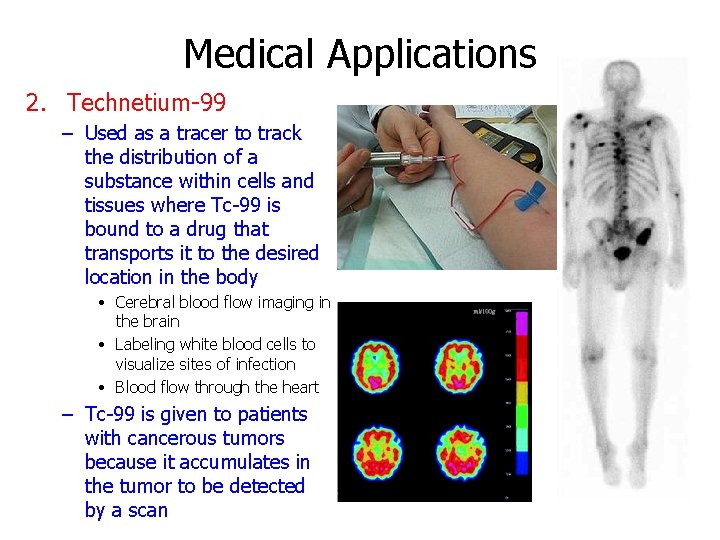
Medical Applications 2. Technetium-99 – Used as a tracer to track the distribution of a substance within cells and tissues where Tc-99 is bound to a drug that transports it to the desired location in the body • Cerebral blood flow imaging in the brain • Labeling white blood cells to visualize sites of infection • Blood flow through the heart – Tc-99 is given to patients with cancerous tumors because it accumulates in the tumor to be detected by a scan
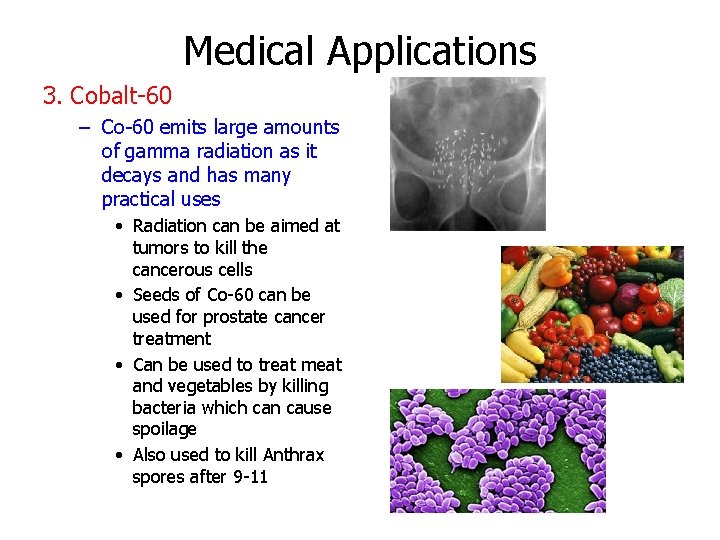
Medical Applications 3. Cobalt-60 – Co-60 emits large amounts of gamma radiation as it decays and has many practical uses • Radiation can be aimed at tumors to kill the cancerous cells • Seeds of Co-60 can be used for prostate cancer treatment • Can be used to treat meat and vegetables by killing bacteria which can cause spoilage • Also used to kill Anthrax spores after 9 -11

Radiation Risks • All of these radioisotopes that are used inside the body need to have very short half lives so that they can be eliminated from the body without destroying healthy tissue. • Potential of damaging normal tissue – Could cause serious illness and death – Could cause mutations that could be passed from generation to generation

Radiation Risks • Decay products from old fuel rods of nuclear reactors are difficult to store and dispose – Was sent to Yucca Mountain Nuclear Waste Repository (defunded by Obama administration in 2011) – Now sent to WIPP (Waste Isolation Power Plant) in New Mexico • Danger posed by nuclear power plants – Accidents could release radioactivity into the air or water
- Slides: 54