Unit 4 Nuclear Chemistry 4 4 Half Life

- Slides: 7

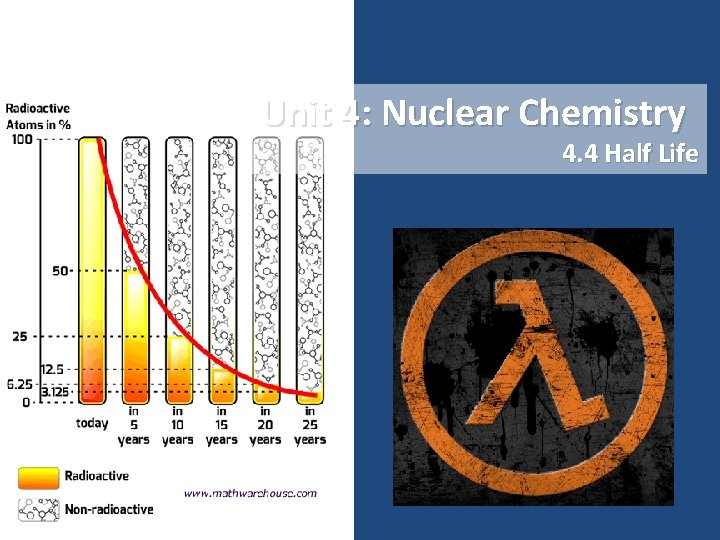
Unit 4: Nuclear Chemistry 4. 4 Half Life

Nuclear Decay • Nuclear Decay is spontaneous – Predicting when a certain atom will decay is impossible – However, we can use statistics to calculate how long it takes a sample to decay • Half-Life – Amount of time it takes for half of the atoms in a sample to decay Radioisotope Radiation Emitted Half-life Kr-94 Beta 1. 4 seconds Rn-222 Alpha 3. 8 days I-131 Beta 8 days Co-60 Gamma 5. 2 years H-3 Beta 12. 3 years C-14 Beta 5, 730 years U-235 Alpha 4. 5 billion years

Half-Life • Example: You start with 100. 0 g sample of a radioactive isotope. How much is left after 1 half-life? After 2 halflives? After 3 half-lives? • Example: The half-life of cobalt-60 is 5. 3 years. If you start with a 1. 000 mg sample how much is left after 15. 9 years? – 15. 9 years = 3 half-lives

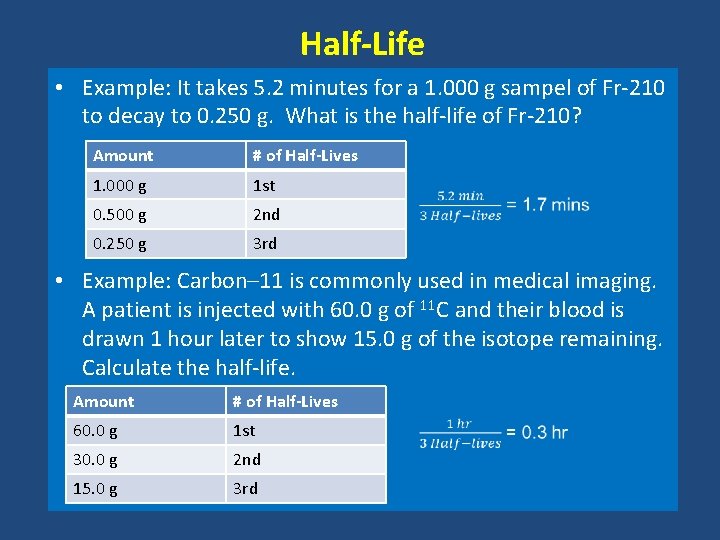
Half-Life • Example: It takes 5. 2 minutes for a 1. 000 g sampel of Fr-210 to decay to 0. 250 g. What is the half-life of Fr-210? Amount # of Half-Lives 1. 000 g 1 st 0. 500 g 2 nd 0. 250 g 3 rd • Example: Carbon– 11 is commonly used in medical imaging. A patient is injected with 60. 0 g of 11 C and their blood is drawn 1 hour later to show 15. 0 g of the isotope remaining. Calculate the half-life. Amount # of Half-Lives 60. 0 g 1 st 30. 0 g 2 nd 15. 0 g 3 rd

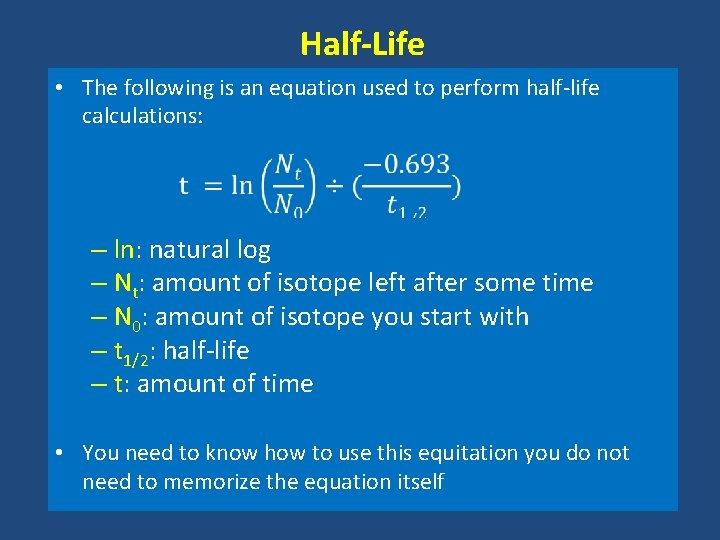
Half-Life • The following is an equation used to perform half-life calculations: – ln: natural log – Nt: amount of isotope left after some time – N 0: amount of isotope you start with – t 1/2: half-life – t: amount of time • You need to know how to use this equitation you do not need to memorize the equation itself

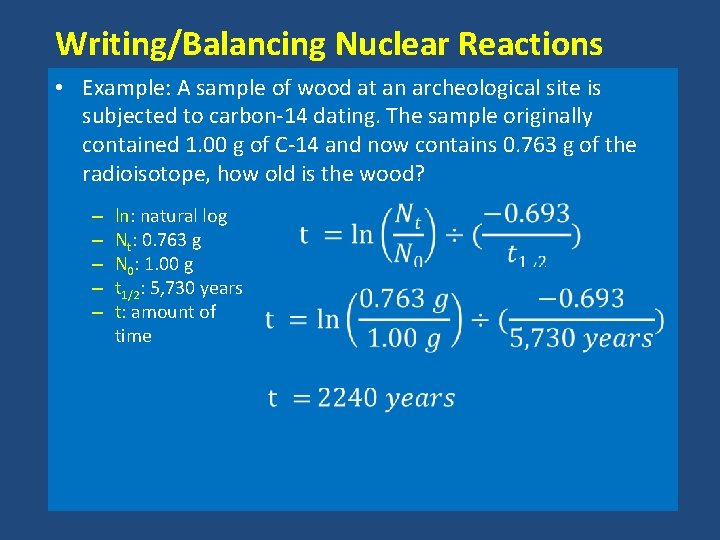
Writing/Balancing Nuclear Reactions • Example: A sample of wood at an archeological site is subjected to carbon-14 dating. The sample originally contained 1. 00 g of C-14 and now contains 0. 763 g of the radioisotope, how old is the wood? – – – ln: natural log Nt: 0. 763 g N 0: 1. 00 g t 1/2: 5, 730 years t: amount of time

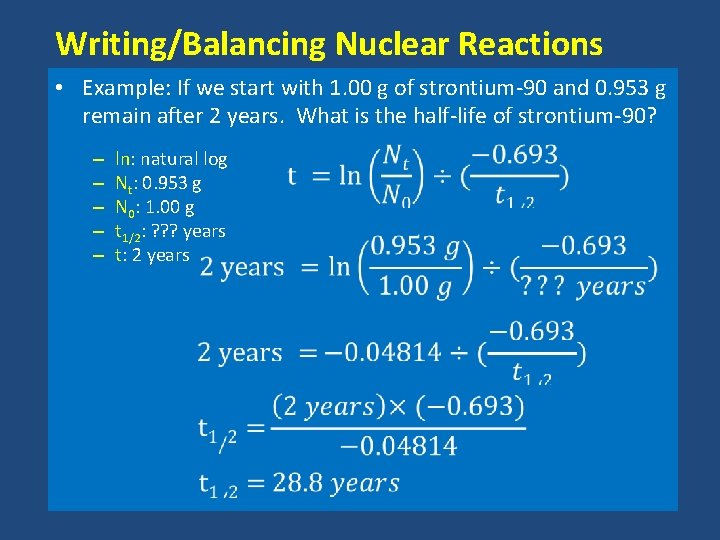
Writing/Balancing Nuclear Reactions • Example: If we start with 1. 00 g of strontium-90 and 0. 953 g remain after 2 years. What is the half-life of strontium-90? – – – ln: natural log Nt: 0. 953 g N 0: 1. 00 g t 1/2: ? ? ? years t: 2 years