Unit 4 Equilibrium Physical and Chemical Equilibria Up

Unit 4 Equilibrium

Physical and Chemical Equilibria • Up to now, we’ve studied only forward reactions. But, there is a possibility for reversible reactions. • Reversible Reaction is where the products revert back to the reactants • We use a double-ended arrow to signify reversible reactions. ex: or

• An equilibrium is established when there is a balance between the forward rate and the reverse rate • In other words, an equilibrium is a balance between two equal and opposing forces (or processes) • When a system reaches equilibrium, it does not undergo any additional changes unless the equilibrium is somehow disturbed. • A Saturated Solution is in equilibrium because there is a balance between the two opposing forces of dissolving and crystallizing.

• This is an example of a physical equilibrium because dissolving and crystallizing are physical changes. ex: Ca. Cl 2(s) Ca 2+(aq) + 2 Cl-(aq) • Just like solubility, such things as temperature, pressure, volume, and concentration can affect an equilibrium. • States of matter (solid, liquid, gas, and aqueous) are incredibly important • Whenever you write equations you must include the states of matter.

• Chemical equilibrium must involve some chemical changes like changes in and pressure. ex: N 2 O 4(g) NO 2(g) • Temperature affects this equilibrium because at low temperatures N 2 O 4(g) exists and at higher temperatures NO 2(g) exists • This equilibrium represents smog so in colder climates, you get the N 2 O 4 which you can not see whereas in warmer climates (like in Mexico City) you get the brown NO 2 which can be seen as a haze over the city

Heterogenous and Homogenous Equilibria • Homogeneous Equilibrium: reactions in which all reacting species are in the same phase ex: N 2 O 4(g) 2 NO 2(g) • Heterogeneous Equilibrium: results from a reversible reaction involving reactants and products that are in different phases ex: Ca. CO 3(s) Ca. O(s) + CO 2(g) • With any type of equilibria, we can write an equilibrium law expression (keq)

• This expression is derived from a balanced chemical equation putting the concentration of the products raised to their coefficients over the concentrations of the reactants raised to their coefficients • This can be done for gaseous and aqueous compounds only ex: Write the equil’m expression for: 2 HI(g) H 2(g) + I 2(g) keq = [H 2] [I 2] [HI]2

Using keq • We need keq because it helps us to predict the direction in which a reaction will proceed to reach equilibrium and to calculate the concentrations of reactants and products once equilibrium has been established • There are two different situations when we can use keq:

1) When we are given the concentrations at equilibrium to find keq • Simply plug in the concentrations to the keq formula ex: For the reaction H 2(g) + I 2(g) 2 HI(g) the following were reached at equilibrium at 25 C [H 2] = 0. 00560 M [I 2] = 0. 000590 M [HI] = 0. 0127 M Find keq = __[HI]2__ [H 2][I 2] = (0. 0127)2____ (0. 0056)(0. 00059) = 48. 82
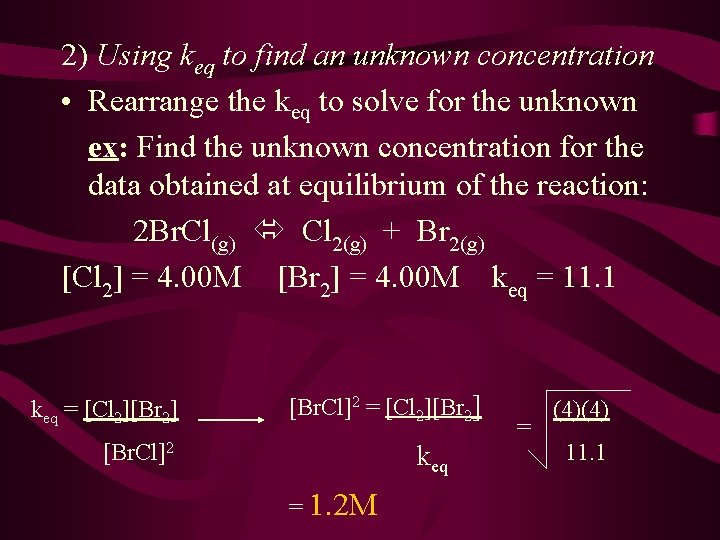
2) Using keq to find an unknown concentration • Rearrange the keq to solve for the unknown ex: Find the unknown concentration for the data obtained at equilibrium of the reaction: 2 Br. Cl(g) Cl 2(g) + Br 2(g) [Cl 2] = 4. 00 M [Br 2] = 4. 00 M keq = 11. 1 keq = [Cl 2][Br 2] [Br. Cl]2 keq = 1. 2 M = (4)(4) 11. 1

• Once we have a value for keq we can predict the favored direction by using: • If keq > 1, then the forward reaction (products) are favored until equilibrium • If keq < 1, then the reverse reaction (reactants) are favored until equilibrium • If keq = 1, then the system is at equilibrium

ICE Charts • We use this method when given initial concentrations • We must set up an “ICE” chart which shows the initial concentrations of all reacting species, the change in concentrations, and the concentrations at equilibrium • We must always have a balanced chemical equation
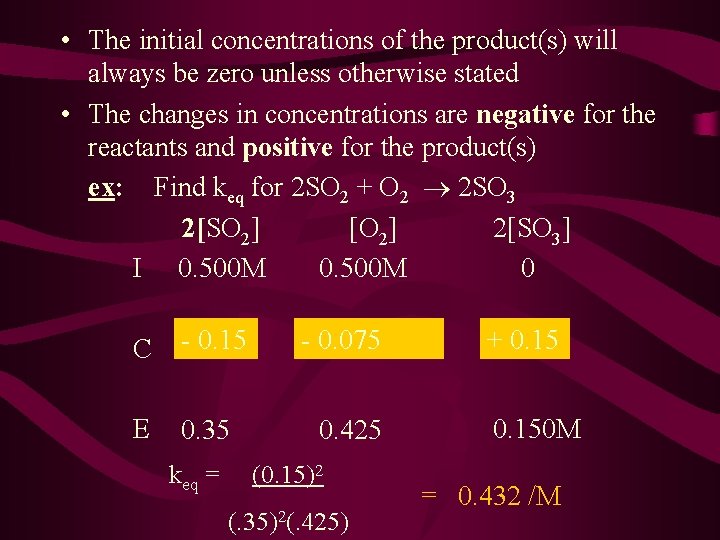
• The initial concentrations of the product(s) will always be zero unless otherwise stated • The changes in concentrations are negative for the reactants and positive for the product(s) ex: Find keq for 2 SO 2 + O 2 2 SO 3 2[SO 2] [O 2] 2[SO 3] I 0. 500 M 0 C - 0. 15 E 0. 35 keq = -(-0. 15 0. 075 x 1)/2 0. 425 (0. 15)2 (. 35)2(. 425) + 0. 150 M = 0. 432 /M

• We can solve for unknown concentrations (if a change is not given) by using the number of moles from each reacting species from the balanced equation • One hint to use this method is that the keq will always be given • The “I” line is done as usual • The “C” line will have x’s based on the balanced equation • If keq is bigger than 0. 1 we will have to square root both sides of the expression • If keq is smaller than 0. 1 we will have to assume any reactant “x” values are zero

ex: 2 HCl H 2 + Cl 2 keq = 16 Find the concentration of chlorine at equilibrium when 20 mol of HCl is placed in a 10 L reaction vessel and allowed to reach equilibrium. I 2 HCl H 2 + Cl 2 2 0 0 C -2 x E 2 – 2 x x x keq = [H 2][Cl 2] [HCl]2 16 = (x)(x) (2 -2 x)2 4 = __x__ 4(2 – 2 x) = x 16 = __x 2__ (2 – 2 x)2 x = 0. 89 M
![ex: For the reaction below, the initial [Q] = 2 M and keq = ex: For the reaction below, the initial [Q] = 2 M and keq =](http://slidetodoc.com/presentation_image_h2/3431813f60eef26c5470a28168f12353/image-16.jpg)
ex: For the reaction below, the initial [Q] = 2 M and keq = 0. 003. Find the concentration of all species at equilibrium. keq = [R]2[T] 3 Q 2 R + T I 2 0 0 C -3 x +2 x +x E 2 -3 x 2 x x 0. 003 = 4 x 3 0. 024 = 4 x 3 [Q]3 0. 003 = (2 x)2(x) (2 -3 x)3 Assign 0 x = 0. 182 M 23 Therefore, [Q]eq = 2 – 3(0. 182) = 1. 45 M, [R]eq = 2(0. 182) = 0. 364 M, and [T]eq = 0. 182 M

Percent Completion • There are 2 ways to use % completion: 1) When a percentage is given: a) multiply the “I” live value by the percentage. The answer goes in the “C” line. b) fill in the rest of the ICE chart the same.
![ex: 3 A + 2 B → 4 C Initially [A] = [B] = ex: 3 A + 2 B → 4 C Initially [A] = [B] =](http://slidetodoc.com/presentation_image_h2/3431813f60eef26c5470a28168f12353/image-18.jpg)
ex: 3 A + 2 B → 4 C Initially [A] = [B] = 3 M. 60% of A is used up. Find keq. 3 A C 3 x 0. 6 -1. 8 E 1. 2 I 2 B 4 C 3 0 -1. 2 1. 8 keq = __(2. 4)4___ (1. 2)3(1. 8)2 +2. 4 = 5. 93 M-1

2) When asked to find % completion a) set up ICE chart normally b) you must find the max possible [prod] i) take the biggest “I” line number and do a mol-mol for the product ii) this is your “max [prod]” c) use the formula: % Comp = act [prod] x 100 max [prod]
![ex: Initially [N 2] = 2 M and [H 2] = 5 M. At ex: Initially [N 2] = 2 M and [H 2] = 5 M. At](http://slidetodoc.com/presentation_image_h2/3431813f60eef26c5470a28168f12353/image-20.jpg)
ex: Initially [N 2] = 2 M and [H 2] = 5 M. At equilibrium [NH 3] = 3 M. Find the % completion. [NH ] I N 2 2 3 H 2 5 2 NH 3 0 C -1. 5 -4. 5 +3 E 0. 5 3 Actual [prod] Biggest “I” value 3 Max [prod] = 5 x 2/3 = 3. 33 % comp = _3_ 3. 33 = 91% x 100 [H 2]

Le Chatelier’s Principle • When something disturbs a system at equilibrium, we say that it has been subjected to a STRESS • When this happens, the system or equilibrium shifts to counteract the stress and then a new equilibrium is established • Examples of stresses include temperature, pressure/volume, concentration and catalysts

1) Concentration • If we change the concentration of one substance, all the other substances need to change in order to keep the keq the same • Even though concentrations have changed and a “SHIFT” has occurred, the keq remains the same • Only changes in gases or aqueous compounds will affect the equilibrium • Changes in solids and liquids have NO effect on the equilibrium
![• A cheater way of knowing the [ ] changes is that wherever • A cheater way of knowing the [ ] changes is that wherever](http://slidetodoc.com/presentation_image_h2/3431813f60eef26c5470a28168f12353/image-23.jpg)
• A cheater way of knowing the [ ] changes is that wherever the main stress is, the change on the same side of the arrow is opposite in direction and the changes on the opposite side of the arrow are same in direction. • To predict direction, we always go away from an addition and towards a take-away (in reference to the main stress) ex: A + B C + D Add [A]

ex 2: Cu(s) + 2 HCl(aq) Cu. Cl 2(aq) + H 2(g) a) What happens when [Cu] is increased? NOTHING!!! Cu is a solid b) What happens when [Cu. Cl 2] is decreased?

2) Temperature • When we have a negative H, that means the reaction is exothermic • Therefore, we can re-write the equation with the heat as one of the products • When we have a positive H we have an endothermic reaction and it is written as one of the reactants • Temperature always affects keq. It’s the only factor that will • All other changes follow the same rules as for concentration
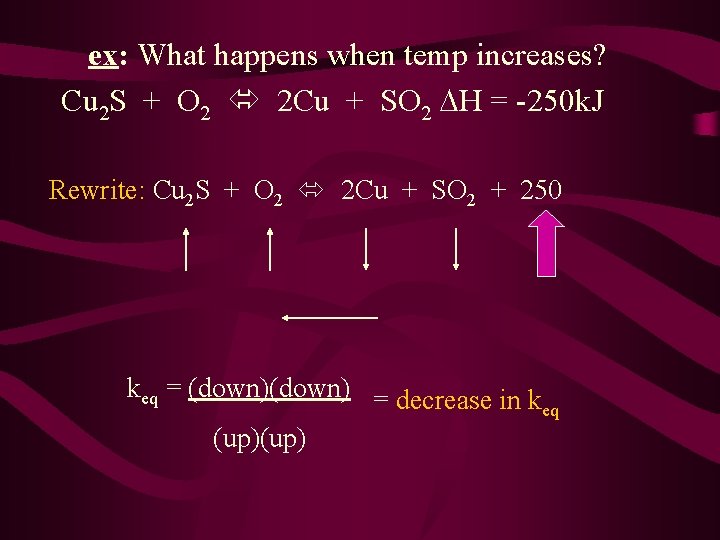
ex: What happens when temp increases? Cu 2 S + O 2 2 Cu + SO 2 H = -250 k. J Rewrite: Cu 2 S + O 2 2 Cu + SO 2 + 250 keq = (down) = decrease in k eq (up)

3) Pressure • Changes in pressure only really affect gases. They have very little effect on solids and liquids • Pressure and volume are inversely related (as one increases the other decreases) • We need to have a balanced chemical equation because we’ll need to count the number of moles (coefficients) on either side (for gases only) • You’ll need to remember three rules:

• If we have an increase in pressure, the reaction will shift to the side that has the fewer number of gas moles • If we have a decrease in pressure, the reaction will shift to the side with more moles of gas • If the number of moles of gas is equal on both sides of the equation, a change in pressure will have no effect • We have to assign the direction arrow first and then the change arrows. • The change arrows will always go up on the side that the direction arrow points to and down on the side that the direction arrow goes away from.
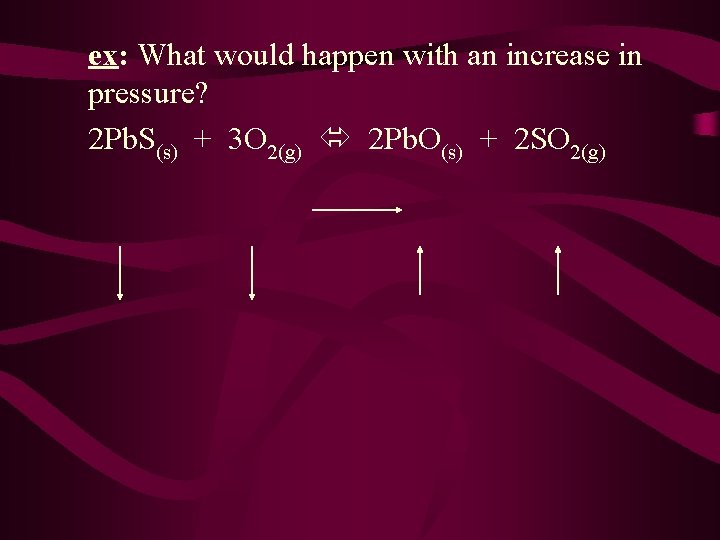
ex: What would happen with an increase in pressure? 2 Pb. S(s) + 3 O 2(g) 2 Pb. O(s) + 2 SO 2(g)

4) Catalysts • Catalysts are NOT considered a stress because they favor neither reaction • They will, however, increase the rate of both the forward and reverse reactions equally. • Therefore, the system will reach equilibrium faster, but it is accomplished WITHOUT A SHIFT • No stress = no effect = no change
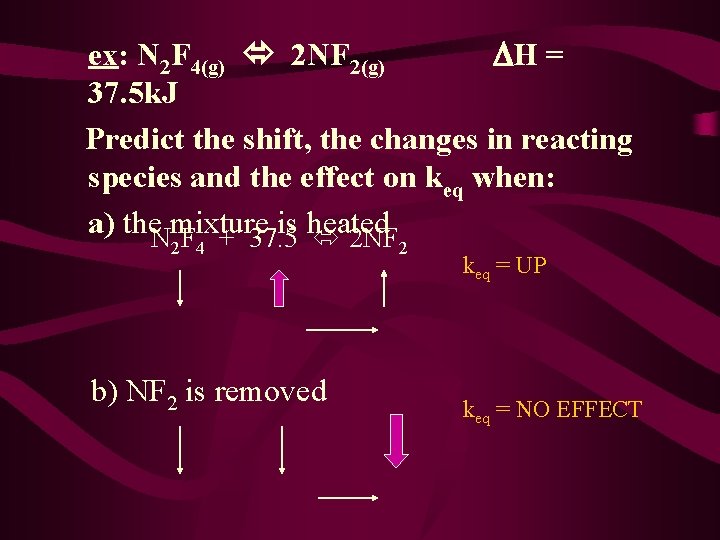
ex: N 2 F 4(g) 2 NF 2(g) H = 37. 5 k. J Predict the shift, the changes in reacting species and the effect on keq when: a) the. Nmixture is heated F + 37. 5 2 NF 2 4 b) NF 2 is removed 2 keq = UP keq = NO EFFECT

c) an inert gas like He is added N 2 F 4 + 37. 5 2 NF 2 NO EFFECT (CATALYST) d) volume is decreased **PRESSURE INCREASE** keq = NO EFFECT
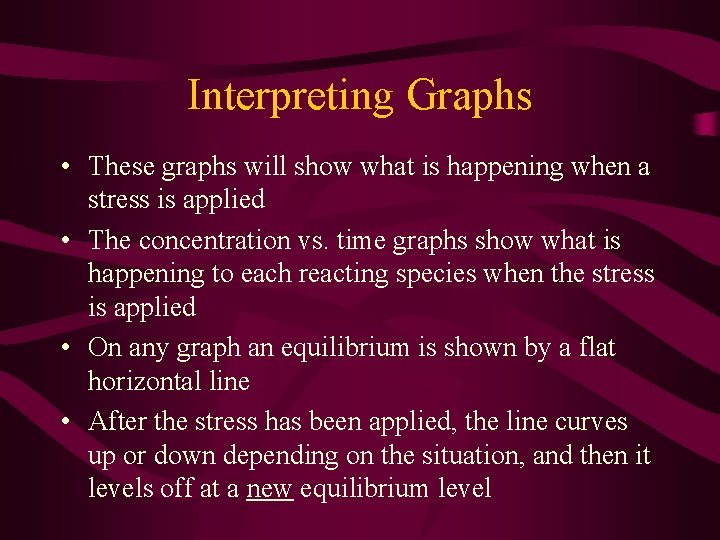
Interpreting Graphs • These graphs will show what is happening when a stress is applied • The concentration vs. time graphs show what is happening to each reacting species when the stress is applied • On any graph an equilibrium is shown by a flat horizontal line • After the stress has been applied, the line curves up or down depending on the situation, and then it levels off at a new equilibrium level

• The stress is indicated by a sharp peak (up for increases and down for decreases) • The rest of the lines gently curve up or down depending on Le Chatelier • Temperature changes cause all reacting species to have gentle curves in the appropriate directions
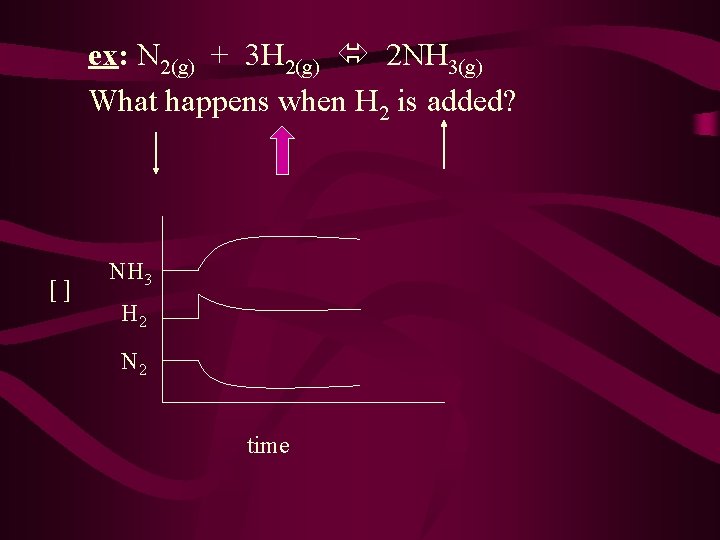
ex: N 2(g) + 3 H 2(g) 2 NH 3(g) What happens when H 2 is added? [] NH 3 H 2 N 2 time

Solubility Product Constant • We need to be able to dissociate a salt into its ions so that we can predict the ion concentrations based on the salt concentration • The salt will always be in the solid state whereas the ions will always be in the aqueous state • The ksp is equal to the product of the concentrations of the ions raised to the power of their respective coefficients
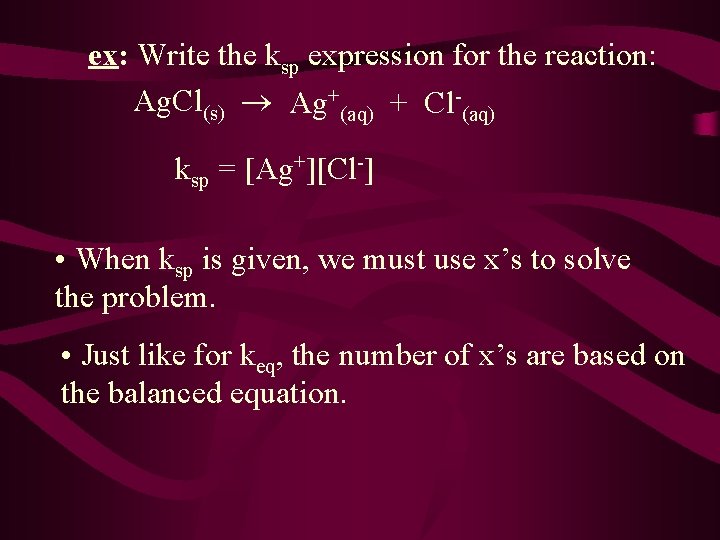
ex: Write the ksp expression for the reaction: Ag. Cl(s) Ag+(aq) + Cl-(aq) ksp = [Ag+][Cl-] • When ksp is given, we must use x’s to solve the problem. • Just like for keq, the number of x’s are based on the balanced equation.

ex: Calcium fluoride has a ksp of 3. 9 x 10 -11. What is the fluoride ion concentration? Ca. F 2(s) Ca 2+(aq) + 2 F-(aq) x 2 x ksp = [Ca 2+][F-]2 3. 9 x 10 -11 = (x)(2 x)2 3. 9 x 10 -11 = 4 x 3 x = 2. 13 x 10 -4 M Therefore, [F-] = 2 x = 2(2. 13 x 10 -4) = 4. 27 x 10 -4 M

ksp vs. TIP • There are times when two salts are added together and we want to know if one salt will dissolve the other • We need to use the ksp in comparison to our experimental values to predict whether a salt precipitate will form when the solutions are mixed (the Trial Ionization Product) • We need to find the final ion concentration of each contributing ion using: Ct = Ci. Vi Vt

• The contributing ions will be the ions that form the substance for which the ksp is given in the question • All other ions are spectator ions • Once you’ve found the Ct for both contributing ions, you multiply them together according to the ksp expression • This experimental result is then compared to our ksp • From the comparison, we can determine: 1) If TIP > ksp a ppt will form 2) If TIP < ksp no ppt will form 3) If TIP = ksp no ppt will form

ex: Predict if a precipitate will form when 15 ml of 1 x 10 -5 M Ba. Cl 2 and 10 ml of 1. 75 x 10 -5 M H 2 SO 4 are mixed (ksp for Ba. SO 4 = 1. 5 x 10 -9) We need to make Ba. SO 4 so we’ll need the Ba 2+ and SO 42 Ba. Cl 2 Ba 2+ + 2 Cl. Ct = (1 x 10 -5)(15) 25 H 2 SO 4 2 H+ + SO 42=6 x 10 -6 Ct = (1. 75 x 10 -5)(10) = 7 x 10 -6 25 TIP = [Ba 2+][SO 42 -] = (6 x 10 -6)(7 x 10 -6) = 4. 2 x 10 -11 M 2 Therefore, since TIP < ksp, no ppt will form!
- Slides: 41