UNIT 4 COVALENT BONDING DAY 8 INTERMOLECULAR FORCES

UNIT 4: COVALENT BONDING DAY 8: INTERMOLECULAR FORCES LAB

WARM UP OPEN TO: Warm Up Section WRITE: Today’s Date DRAW: The following molecules 1. HBr 2. SF 2 3. N 2 TIME: 6 MINUTES WHEN DONE: Set up Cornell Note on next available right hand page

AGENDA • NOTES: INTERMOLECULAR FORCES • EVAPORATION AND INTERMOLECULAR FORCES PRE-LAB • EVAPORATION AND INTERMOLECULAR FORCES LAB NEXT CLASS NOTES: REVIEW COVALENT BONDING UNIT 4 STUDY GUIDE POLAR VS. NON-POLAR ASSIGNMENT ASSESSMENT: TUESDAY MAR 14 TH
UNIT 4 LONG TERM LEARNING TARGET I CAN PREDICT PROPERTIES OF A SUBSTANCE BASED ON ITS MOLECULAR STRUCTURE AND SUPPORT THESE PREDICTIONS WITH EXPERIMENTAL DATA.

SET UP NOTES ON NEXT AVAILABLE RIGHT-HAND PAGE: SET UP CORNELL NOTES TITLE: INTERMOLECULAR FORCES ESSENTIAL QUESTION: WHY DO CERTAIN MOLECULES EVAPORATE FASTER? WHY ARE CERTAIN MOLECULES “WEAKER”? GLUE: IMAGE OF WATER MOLECULES TIME: 3 MINUTES WHEN DONE: THINK, WHAT DOES “INTERMOLECULAR” MEAN?
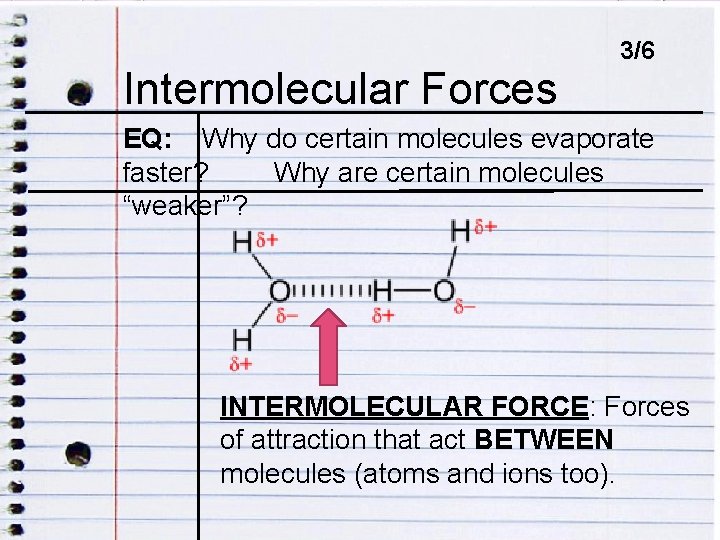
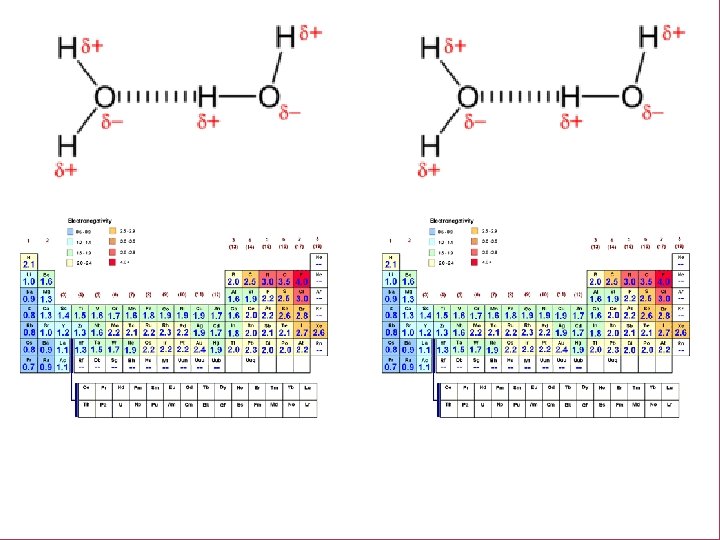
Intermolecular ENDOCRINE DISRUPTORSForces 3/6 EQ: Why do certain molecules evaporate faster? Why are certain molecules EQ: HOW DO ENDOCRINE DISRUPTORS IMPACT “weaker”? HUMANS? INTERMOLECULAR FORCE: Forces of attraction that act BETWEEN molecules (atoms and ions too).
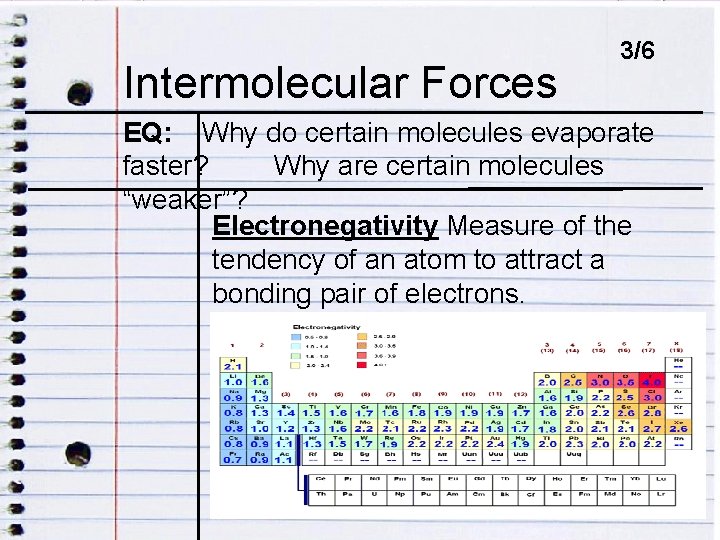
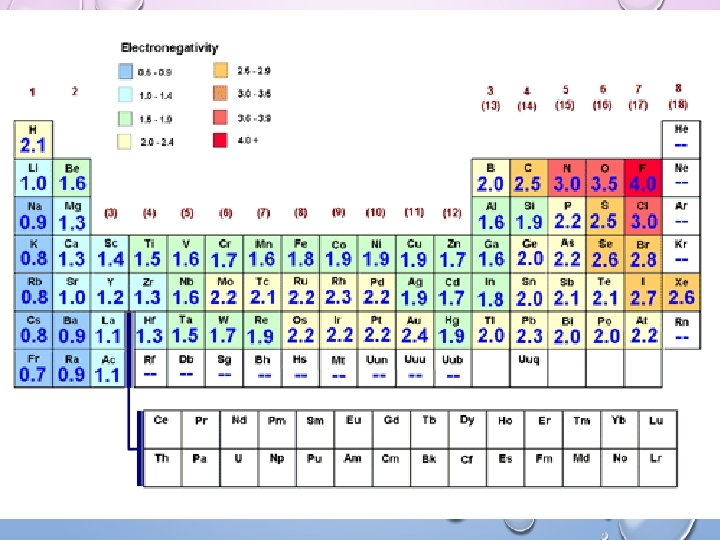
Intermolecular Forces ENDOCRINE DISRUPTORS 3/6 EQ: Why do certain molecules evaporate faster? Why are certain molecules EQ: HOW DO ENDOCRINE DISRUPTORS IMPACT “weaker”? HUMANS? Electronegativity Measure of the tendency of an atom to attract a bonding pair of electrons.

Intermolecular Forces ENDOCRINE DISRUPTORS 3/6 EQ: Why do certain molecules evaporate faster? Why are certain molecules EQ: HOW DO ENDOCRINE DISRUPTORS IMPACT “weaker”? HUMANS? Polar Molecules: Have positive and negative “parts” because electrons in a bond are not shared equally – one atom is more electronegative than the other and pulls the bonding electrons toward it. Non - Polar Molecules: Do not have positive or negative “parts” because the bonding electrons are shared equally or are pulled with equal strength because the molecule is symmetrical
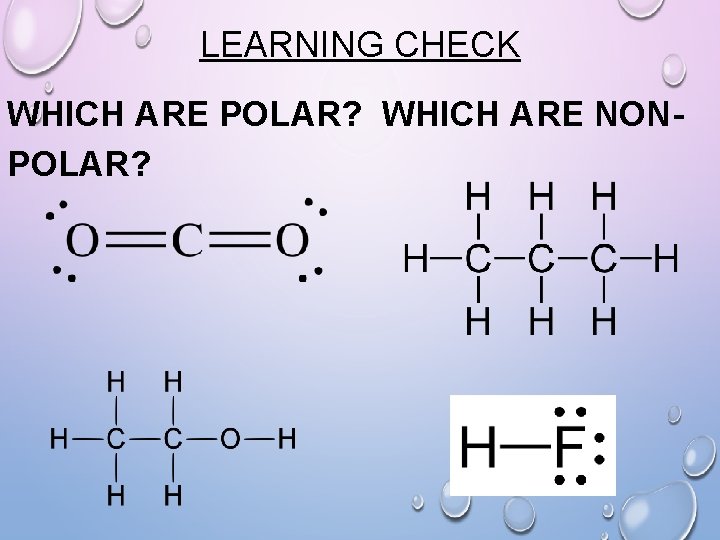
LEARNING CHECK WHICH ARE POLAR? WHICH ARE NONPOLAR?
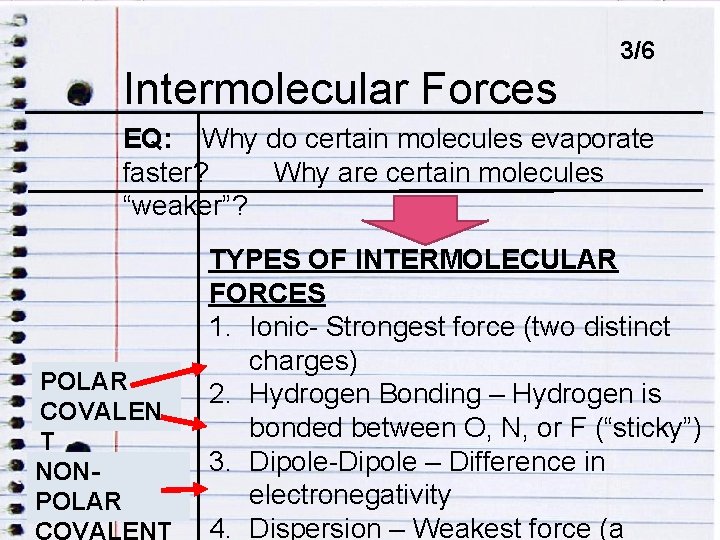
Intermolecular ENDOCRINE DISRUPTORSForces 3/6 EQ: Why do certain molecules evaporate faster? Why are certain molecules EQ: HOW DO ENDOCRINE DISRUPTORS IMPACT “weaker”? HUMANS? POLAR COVALEN T NONPOLAR TYPES OF INTERMOLECULAR FORCES 1. Ionic- Strongest force (two distinct charges) 2. Hydrogen Bonding – Hydrogen is bonded between O, N, or F (“sticky”) 3. Dipole-Dipole – Difference in electronegativity 4. Dispersion – Weakest force (a

WHICH ONE IS STRONGER? METHANE OR METHANOL? METHA NE METHANO L STRONGER! HYDROGEN BONDING
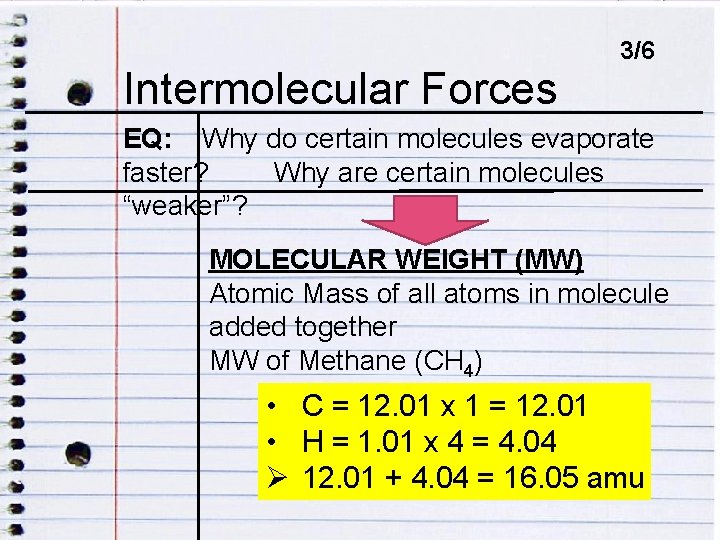
Intermolecular ENDOCRINE DISRUPTORSForces 3/6 EQ: Why do certain molecules evaporate faster? Why are certain molecules EQ: HOW DO ENDOCRINE DISRUPTORS IMPACT “weaker”? HUMANS? MOLECULAR WEIGHT (MW) Atomic Mass of all atoms in molecule added together MW of Methane (CH 4) • C = 12. 01 x 1 = 12. 01 • H = 1. 01 x 4 = 4. 04 Ø 12. 01 + 4. 04 = 16. 05 amu

Intermolecular ENDOCRINE DISRUPTORSForces 3/6 EQ: Why do certain molecules evaporate faster? Why are certain molecules EQ: HOW DO ENDOCRINE DISRUPTORS IMPACT “weaker”? HUMANS? MW Cont Two Molecules with the same intermolecular forces, but the one witha larger molecular weight will be “stronger” Which molecule will evaporate slower? CH 4 MW = 16. 05 amu C 2 H 6 MW = 30. 07 amu Which molecule is “stronger”?

REVIEW YOUR NOTES 1. CHUNK: YOUR NOTES 2. HIGHLIGHT OR CIRCLE: KEY TERMS 3. WRITE: 4 QUESTIONS IN THE MARGINS TIME: 6 MINUTES WHEN DONE: FIND EVAPORATION AND INTERMOLECULAR LAB IN

EVAPORATION AND INTERMOLECULAR LAB FIND: EVAPORATION AND INTERMOLECULAR LAB (IN BLUE BIN) NOTICE: WHICH COMPOUNDS HAVE HYDROGEN BONDING? WHICH DO NOT? (LOOK AT PRE-LAB EXERCISE) TIME: 3 MINUTES WHEN DONE: BE READY TO FILL IN FOLLOW EXAMPLE ON PRE-LAB

EVAPORATION AND INTERMOLECULAR LAB COMPLETE: THE REST OF THE PRELAB WITH YOUR TABLE PARTNERS THEN: READ THROUGH PROCEDURE, PAYING SPECIAL ATTENTION TO DIAGRAMS TIME: 13 MINUTES WHEN DONE: SHOW ME YOUR PRELAB AND BE READY TO REVIEW

EVAPORATION AND INTERMOLECULAR LAB READ: PROCEDURE COMPLETE: THE LAB TIME: 13 MINUTES WHEN DONE: SHOW ME YOUR DATA

- Slides: 20