Unit 4 chapter 4 ATOMIC STRUCTURE Atomic Structure



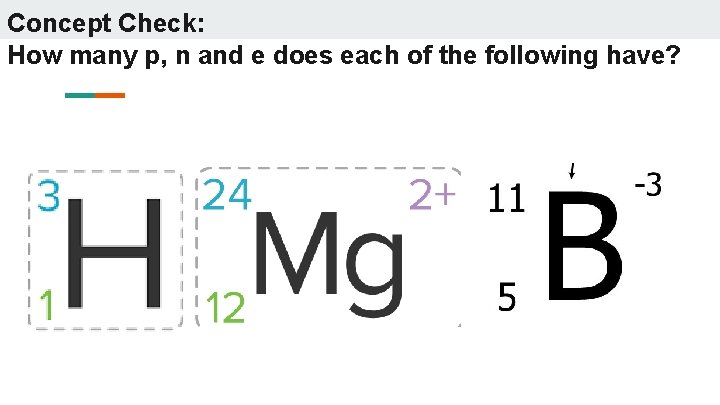




- Slides: 17

Unit 4 (chapter 4) ATOMIC STRUCTURE

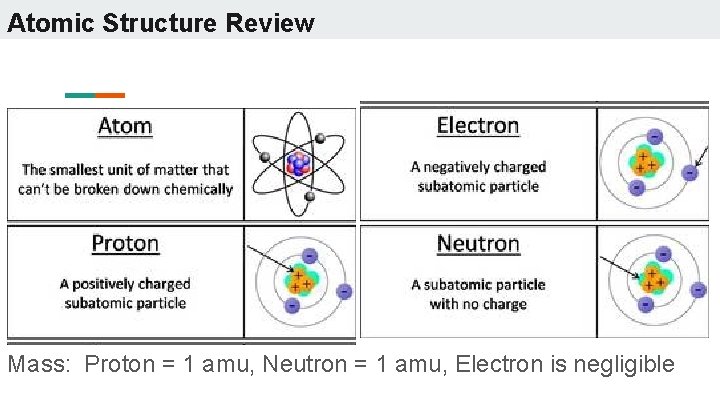
Atomic Structure Review Mass: Proton = 1 amu, Neutron = 1 amu, Electron is negligible


Protons ● ● ● The # of protons is equal to the atomic number Determines the identity of the element Charge of +1 Found: nucleus Symbol: p+ Discovered by Ernest Rutherford in the early 1900’s

Neutrons ● The atomic mass = # p + # n ● Differing numbers of neutrons in the same element are isotopes ● Neutral in charge ● Found: nucleus ● Symbol : n 0 ● Discovered by James Chadwick in 1932

Electrons ● The #electrons = # protons in a neutral atom ● If the # electrons ≠ # protons, then it is an ion ● + ion = cation ● - ion = anion ● Charge of -1 ● Found: orbitals ● Symbol : e● Discovered by J. J. Thomson in 1897.

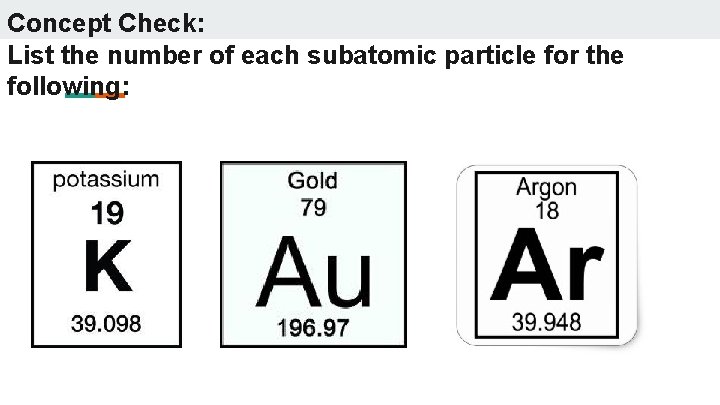
Concept Check: List the number of each subatomic particle for the following:

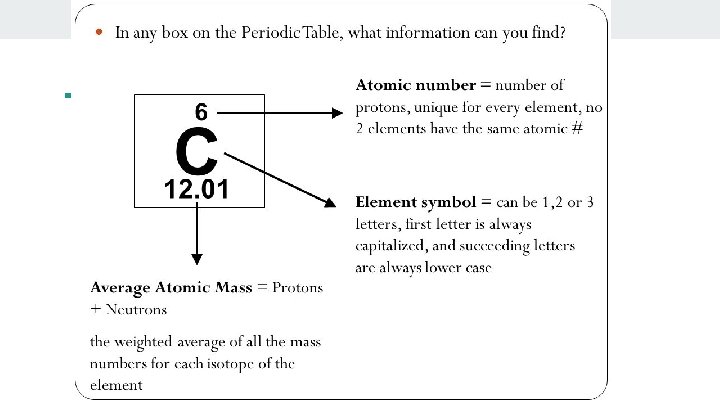
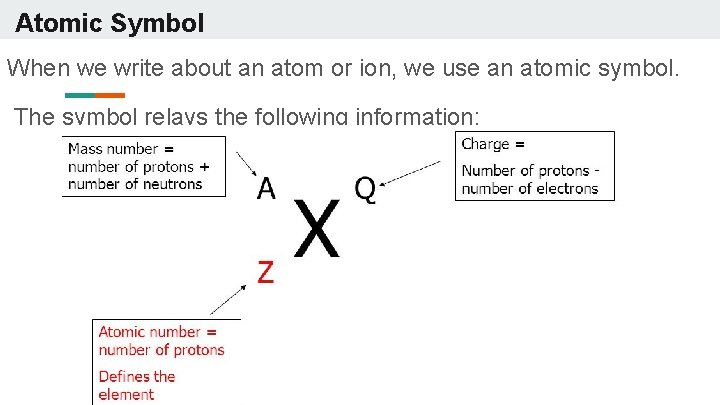
Atomic Symbol When we write about an atom or ion, we use an atomic symbol. The symbol relays the following information:
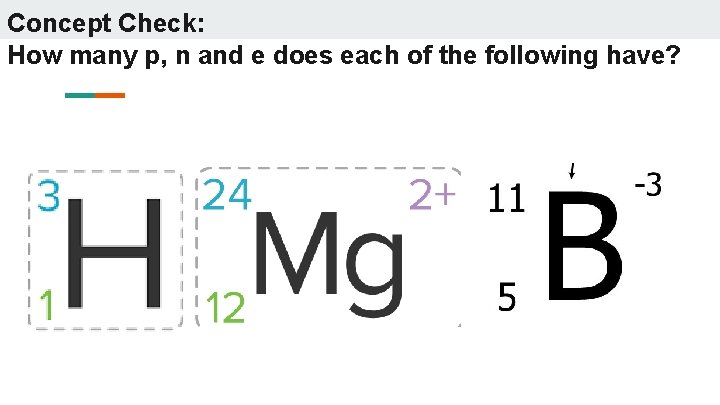
Concept Check: How many p, n and e does each of the following have?

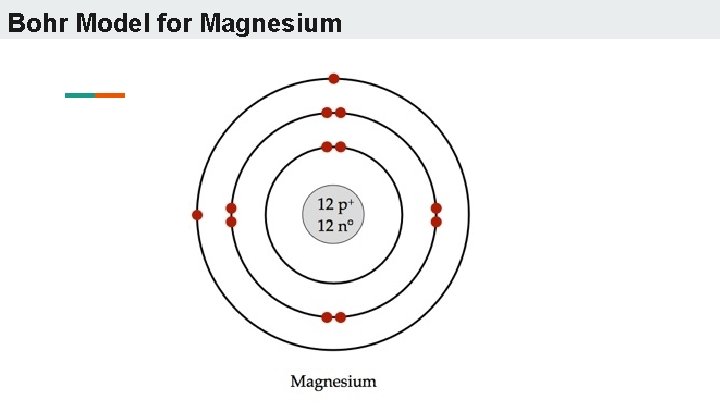
Bohr Model for Magnesium

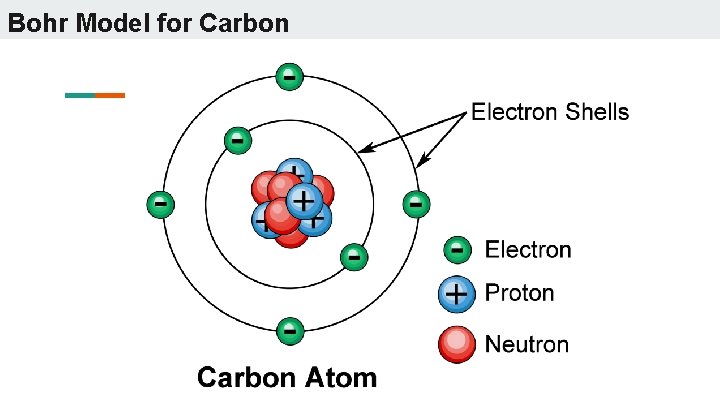
Bohr Model for Carbon

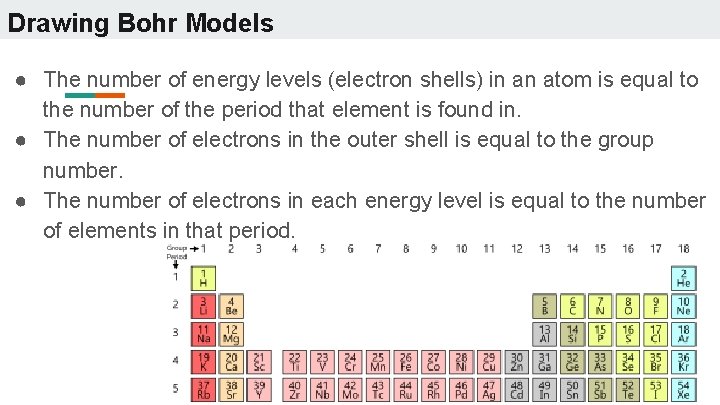
Drawing Bohr Models ● The number of energy levels (electron shells) in an atom is equal to the number of the period that element is found in. ● The number of electrons in the outer shell is equal to the group number. ● The number of electrons in each energy level is equal to the number of elements in that period.

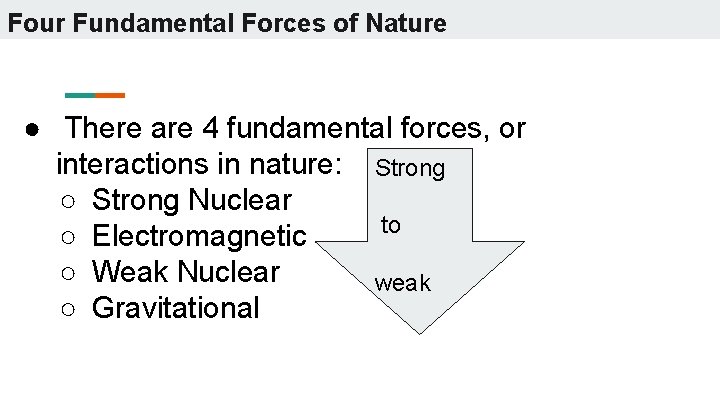
Four Fundamental Forces of Nature ● There are 4 fundamental forces, or interactions in nature: Strong ○ Strong Nuclear to ○ Electromagnetic ○ Weak Nuclear weak ○ Gravitational

Strong Nuclear Force ● Holds the nuclei of atoms together ● Very strong, but only over very, very short distances (within the nucleus of the atom)

Electromagnetic Forces ● Causes electric and magnetic effects ● Like charges repel each other ● Opposite charges attract each other ● Weaker than the strong nuclear force ● Acts over a much longer distance range than the strong nuclear force

Weak Nuclear Force ● Responsible for nuclear decay- changes one nucleon into another ● Weak and has a very short distance range (nucleons must be touching)

Gravitational Force ● Weakest of all fundamental forces, but acts over very long distances ● Attractive force between any two pieces of matter in the universe ● Very important in explaining the structure of the universe