Unit 3 Vocabulary Terms Atomic Theory The theory

Unit 3 Vocabulary Terms
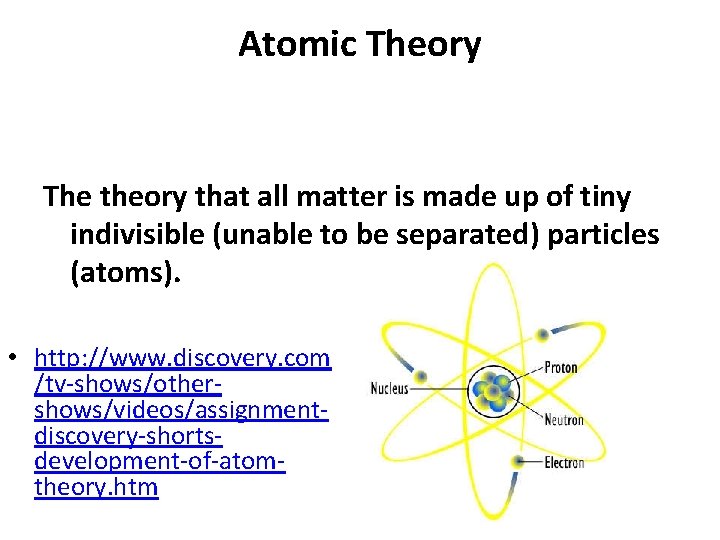
Atomic Theory The theory that all matter is made up of tiny indivisible (unable to be separated) particles (atoms). • http: //www. discovery. com /tv-shows/othershows/videos/assignmentdiscovery-shortsdevelopment-of-atomtheory. htm
Atom • The basic unit of a chemical element https: //www. youtube. com/w atch? v=o-3 I 1 JGW-Ck
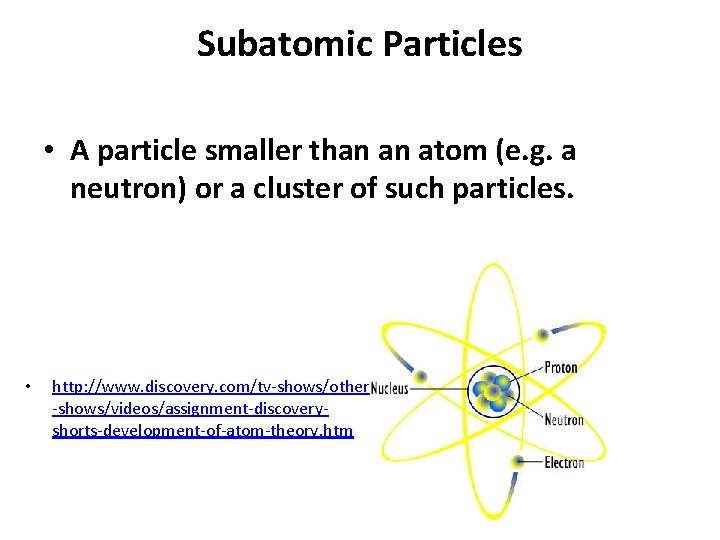
Subatomic Particles • A particle smaller than an atom (e. g. a neutron) or a cluster of such particles. • http: //www. discovery. com/tv-shows/other -shows/videos/assignment-discoveryshorts-development-of-atom-theory. htm

Proton • A positively charged subatomic particle that is found in the nucleus of an atom. http: //media-cacheak 0. pinimg. com/originals/98/43 /e 5/9843 e 5367 effb 0340 a 84 c 81 5 adc 872 f 6. jpg

Neutron • A neutral subatomic particle that is found in the nucleus of an atom. http: //cartoonpicturecollection. blog spot. com/2010/03/jimmy-neutronbest-wallpaper-gallery. html
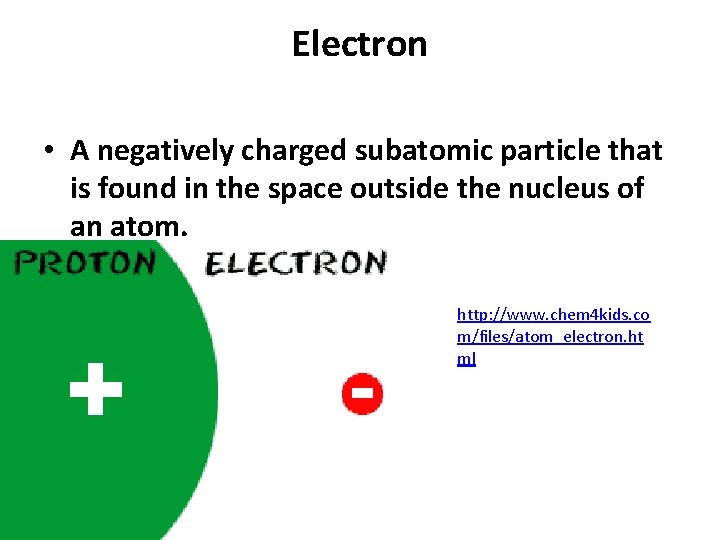
Electron • A negatively charged subatomic particle that is found in the space outside the nucleus of an atom. http: //www. chem 4 kids. co m/files/atom_electron. ht ml
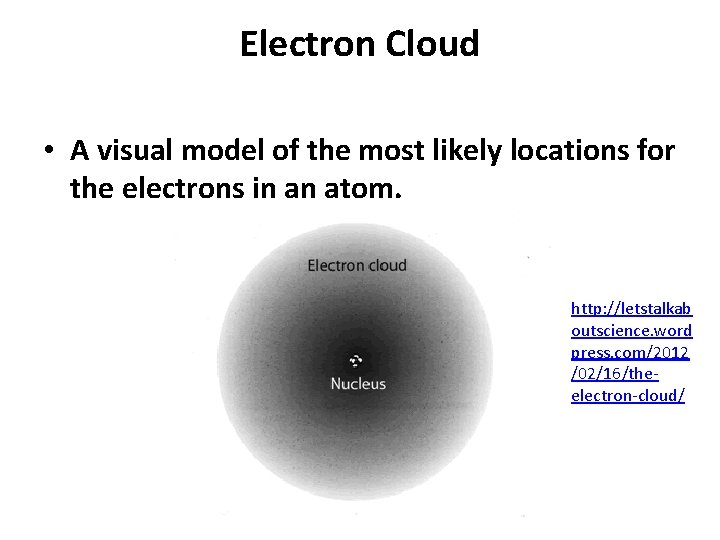
Electron Cloud • A visual model of the most likely locations for the electrons in an atom. http: //letstalkab outscience. word press. com/2012 /02/16/theelectron-cloud/
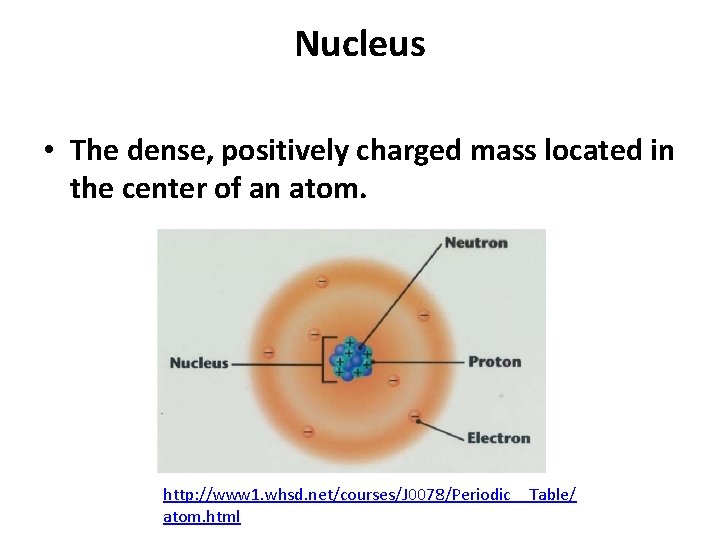
Nucleus • The dense, positively charged mass located in the center of an atom. http: //www 1. whsd. net/courses/J 0078/Periodic__Table/ atom. html
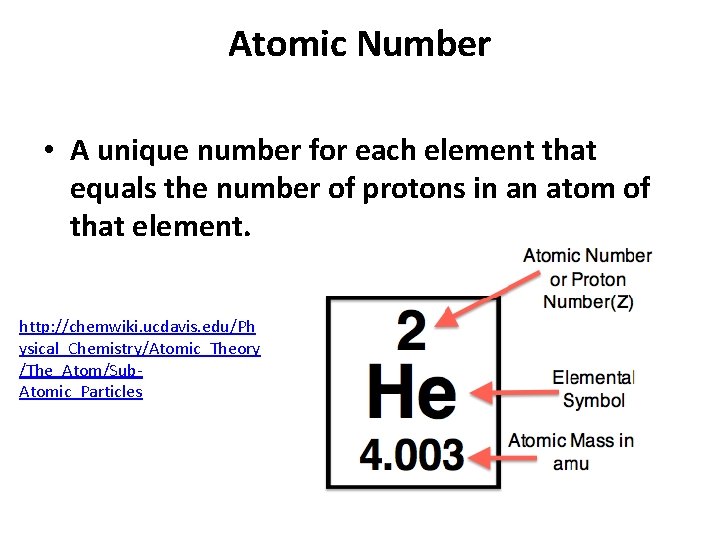
Atomic Number • A unique number for each element that equals the number of protons in an atom of that element. http: //chemwiki. ucdavis. edu/Ph ysical_Chemistry/Atomic_Theory /The_Atom/Sub. Atomic_Particles
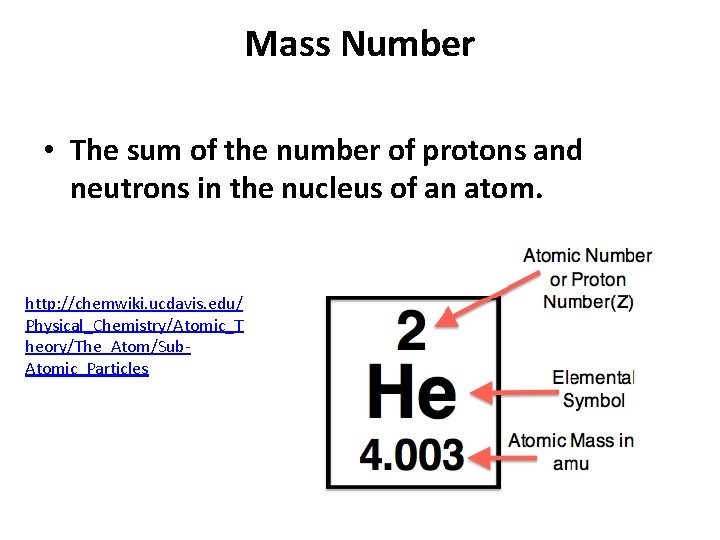
Mass Number • The sum of the number of protons and neutrons in the nucleus of an atom. http: //chemwiki. ucdavis. edu/ Physical_Chemistry/Atomic_T heory/The_Atom/Sub. Atomic_Particles
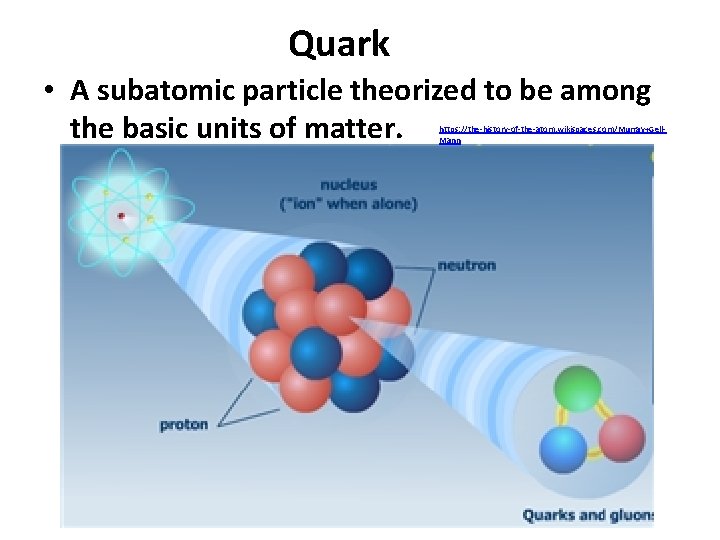
Quark • A subatomic particle theorized to be among the basic units of matter. https: //the-history-of-the-atom. wikispaces. com/Murray+Gell. Mann
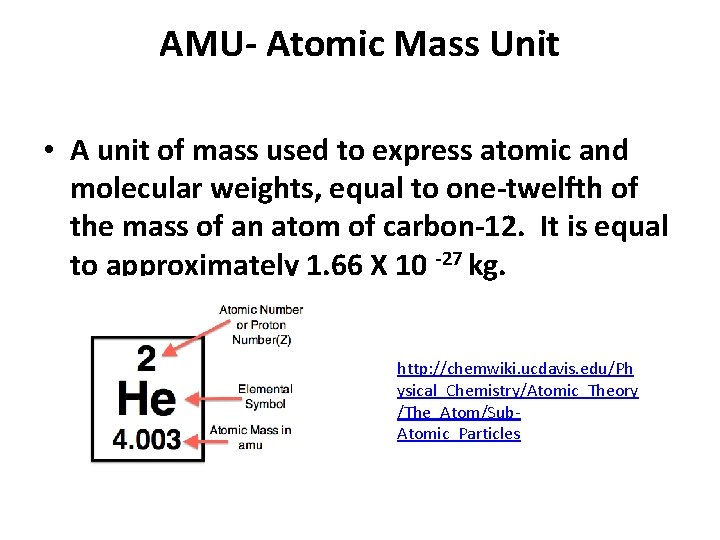
AMU- Atomic Mass Unit • A unit of mass used to express atomic and molecular weights, equal to one-twelfth of the mass of an atom of carbon-12. It is equal to approximately 1. 66 X 10 -27 kg. http: //chemwiki. ucdavis. edu/Ph ysical_Chemistry/Atomic_Theory /The_Atom/Sub. Atomic_Particles
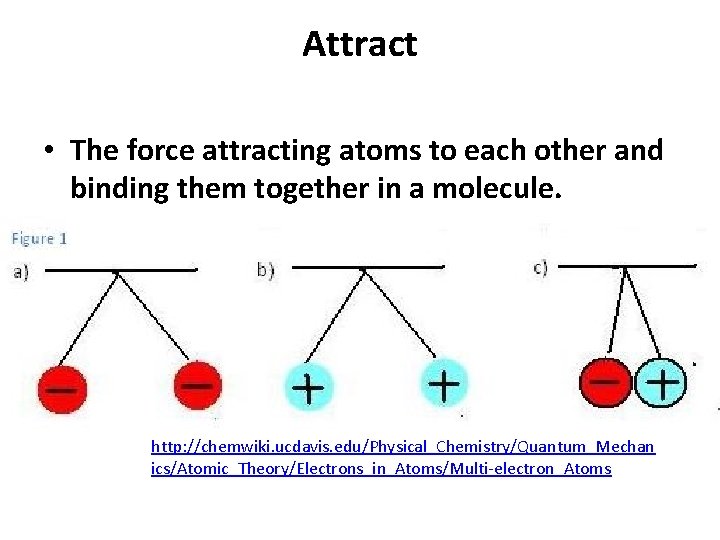
Attract • The force attracting atoms to each other and binding them together in a molecule. http: //chemwiki. ucdavis. edu/Physical_Chemistry/Quantum_Mechan ics/Atomic_Theory/Electrons_in_Atoms/Multi-electron_Atoms

Crash Course Video • https: //www. youtube. com/watch? v=thn. Dx. Fd kz. Zs

1. A negatively charged subatomic particle that is found in the space outside the nucleus of an atom. A. B. C. D. Electron Proton Neutron Quark

2. The theory that all matter is made up of tiny indivisible (unable to be separated) particles (atoms). A. B. C. D. Subatomic Particles Atomic Number AMU Atomic Theory

3. A particle smaller than an atom or cluster of such particles. A. B. C. D. Atom Quark Subatomic Particles Mass Number

4. A visual model of the most likely locations for the electrons in an atom. A. B. C. D. Atomic Theory Electron Cloud Mass Number Quark
5. A unit of mass used to express atomic and molecular weights, equal to one-twelfth of the mass of an atom of carbon-12 A. B. C. D. AMU- Atomic Mass Unit Mass Number Atomic Electron

6. The sum of the number of protons and neutrons in the nucleus of an atom. A. B. C. D. AMU Proton Atomic Theory Mass Number

7. A unique number for each element that equals the number of protons in an atom of that element. A. B. C. D. Mass Number Atomic Number Electrons Protons
- Slides: 22