UNIT 3 THE ATOM The Atom Smallest unit
























- Slides: 30

UNIT 3 THE ATOM

The Atom � � Smallest unit of matter Three subatomic particles � Protons � Neutrons � Electrons

Protons � � Positively charged Are part of the nucleus Have a mass of 1 amu Number of protons = atomic number � Defines the atom

Neutrons � � Have a neutral charge Also part of the nucleus Have a mass of 1 amu neutrons + protons = atomic mass

Electrons � � Have a negative charge Moving around the nucleus Have little mass Number of electrons = number of protons

Isotopes � Variant forms of an atom � Atoms with same number of protons (C = 6) have different number of neutrons 12 C has 6 protons and 6 neutrons; 14 C has 6 protons and 8 neutrons. � Some isotopes (radioactive) are unstable and decay into more stable atoms Used to date rocks and fossils Used as tracers to follow atom through reactions or through body

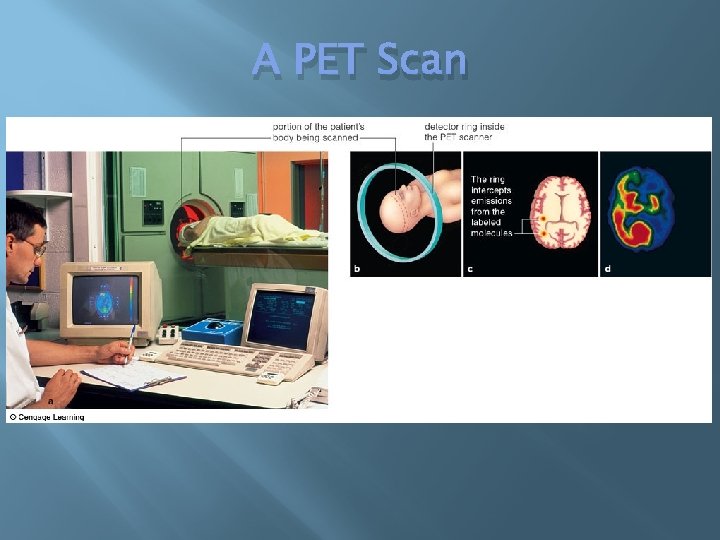
Using Radioisotopes to Track Chemicals & Save Lives � � Radioisotopes are radioactive isotopes They are not stable � Emit particles and energy as they decay spontaneously into other elements � Decays at a constant rate into the same products Example: 14 C → 14 N � Tracer � Molecule with a detectable substance attached � PET scans

A PET Scan

Why Electrons Matter � � Electrons & Energy Levels Full Shells = Happy Shells Unfilled Shells = Reactive Atoms Molecules

Electrons & Energy Levels � � Electrons are attracted to protons but are repelled by other electrons Orbitals are volumes of space around nucleus where electrons are found � Each � orbital hold 2 electrons Shells � Are levels or orbitals � Closest to nucleus = 1 orbital = 2 electrons � Next shell = 4 orbitals = 8 electrons

Full Shells � � Any atom with a vacant orbital will tend to fill it by forming a bond with other atom(s) Chemical bonds are unions between electron structures of atoms

Unfilled Shells � May give up , gain, or share electrons � Chemical bond � Distribution of electrons changes if the atom gives up, gains, or shares electron(s)

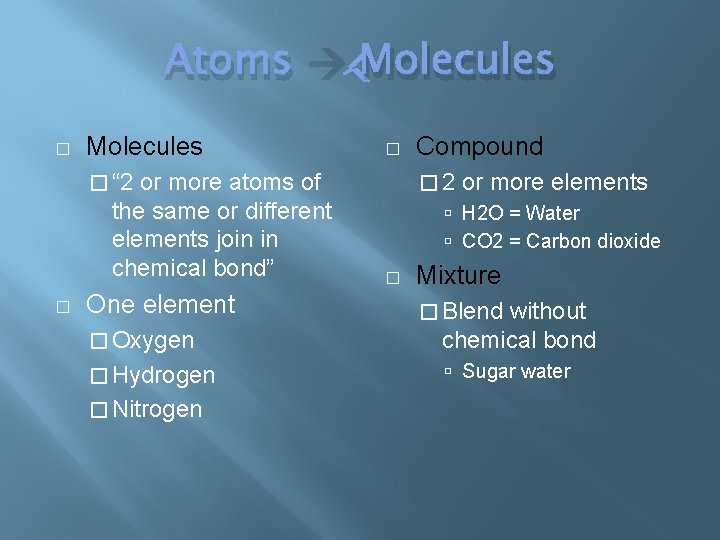
Atoms Molecules � Molecules or more atoms of the same or different elements join in chemical bond” � � “ 2 � One element Compound � 2 or more elements H 2 O = Water CO 2 = Carbon dioxide � Mixture � Oxygen without chemical bond � Hydrogen Sugar water � Nitrogen � Blend

What Happens When Atoms Bond � � � Ionic Bond Covalent Bond Hydrogen Bond

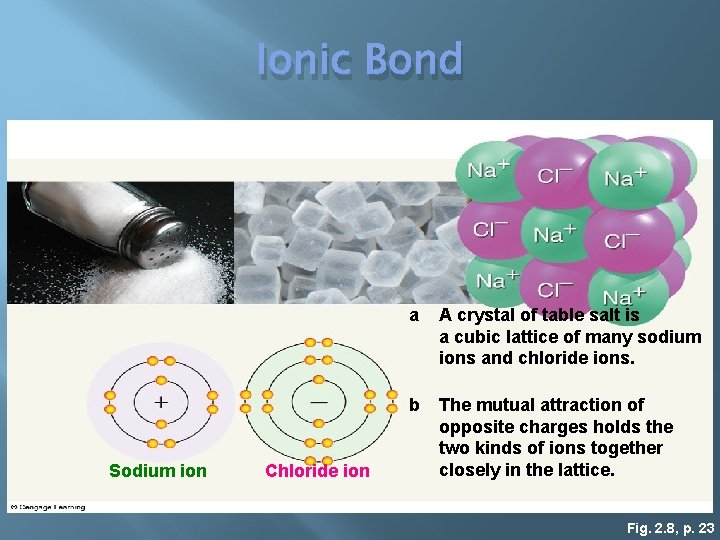
Ionic Bond � Electrons are transferred � Gained � Lost � Forms ions atoms with a net electrical charge +/� Gain electrons = - charge � Lose electrons = + charge � Positive and negative ions attract each other � Na. Cl � HCl � KBr

Ionic Bond Sodium ion Chloride ion a A crystal of table salt is a cubic lattice of many sodium ions and chloride ions. b The mutual attraction of opposite charges holds the two kinds of ions together closely in the lattice. Fig. 2. 8, p. 23

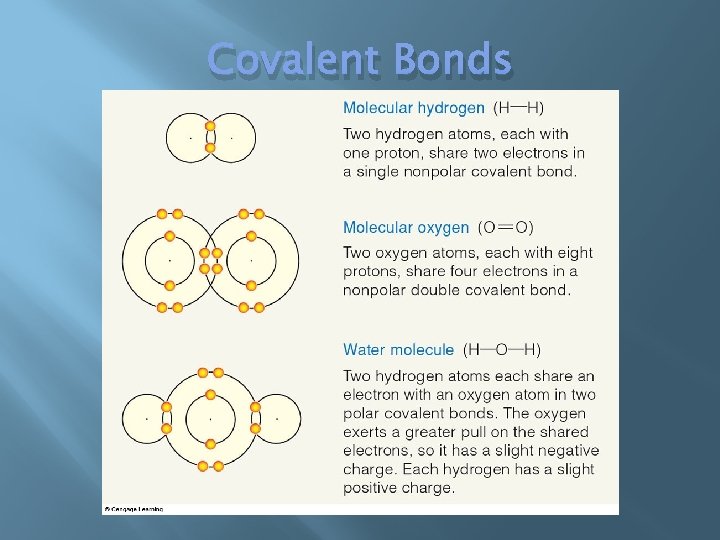
Covalent Bond � � Electrons shared Non-polar covalent � Equally � shared Polar covalent � Unequally shared � Slight positive & negative charge to opposite “poles” of the molecule � Charge areas attract Hydrogen bond

Covalent Bonds

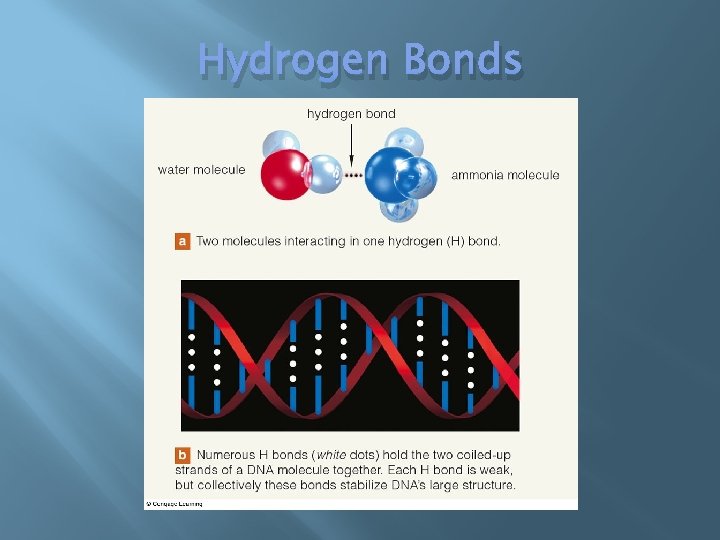
Hydrogen Bonds � Form between a hydrogen atom and an electronegative atom � Each � with separate polar covalent bonds Are not chemical bonds � Do not make atoms into molecules � Individually weak � Collectively stabilize structures of large molecules

Hydrogen Bonds

Properties of Water Molecules � Water molecules are polar � Form hydrogen bonds with other polar molecules � Hydrophilic substances (water-loving) � Hydrophobic substances (water-dreading)

Water’s Life-Giving Properties � Polarity gives liquid water unique properties that make life possible: � Resistance to temperature changes � Internal cohesion � Dissolves polar and ionic substances

High Heat Capacity � � � Absorbs heat without changing temperature Evaporation = cooling Freezing hydrogen bonds resist breaking � Crystal structure is less dense than water

Cohesion � Hydrogen bonding � Cohesion � (stickiness) Cohesion pulls water through plants � Capillary action

Water as a Solvent � � � Solvent is a substance, usually liquid, that dissolves other substances Solutes are dissolved substances Water is an excellent solvent � Will dissolve most polar molecules � Is the most nearly universal solvent � Form spheres of hydrogen Clusters of water molecules around a solute A substance is said to be dissolved after solvent molecules cluster around its ions or molecules & keep them disapersed

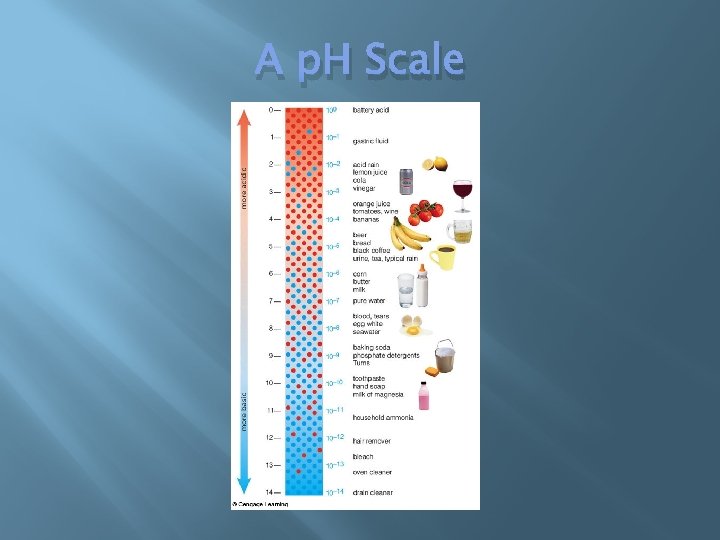
p. H Scale � � � Arrange substances from acid to base 0 -14 7 = Neutral Power of hydrogen (negative exponent)

A p. H Scale

Acids & Bases � Acids 0 -7 on p. H scale � (Protons) H+ ions � Red in litmus � Sour taste � React with metals � Bases 7 -14 on p. H scale � OH- ion � Blue in litmus � Bitter taste

Buffers � Weak acid and the base that forms in water � H 2 CO 3 HCO 3 - + H+ � Carbonic acid Bicarbonate � Prevents swings in p. H.

Salts � Form from an acid and a base � Hydrochloric acid + Sodium hydroxide = Sodium chloride + water � HCl + Na. OH Na. Cl + H 2 � Useful ions in solution