Unit 3 Rates and Chemical Equilibrium CH 1121

Unit 3: Rates and Chemical Equilibrium CH 1121
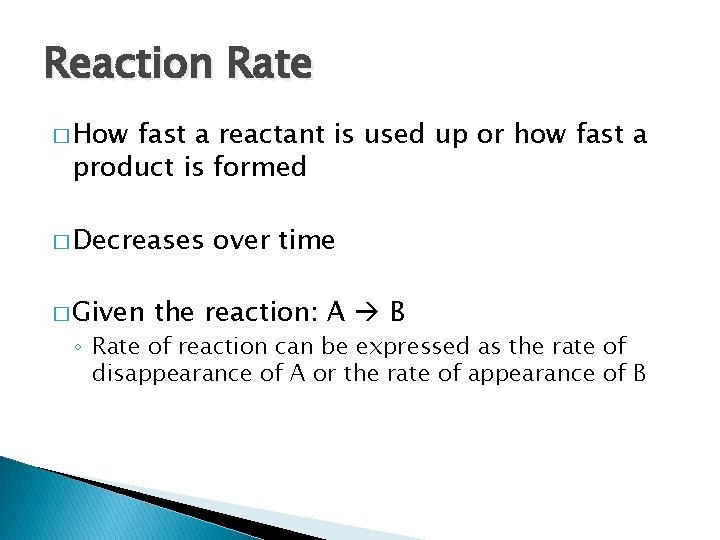
Reaction Rate � How fast a reactant is used up or how fast a product is formed � Decreases � Given over time the reaction: A B ◦ Rate of reaction can be expressed as the rate of disappearance of A or the rate of appearance of B
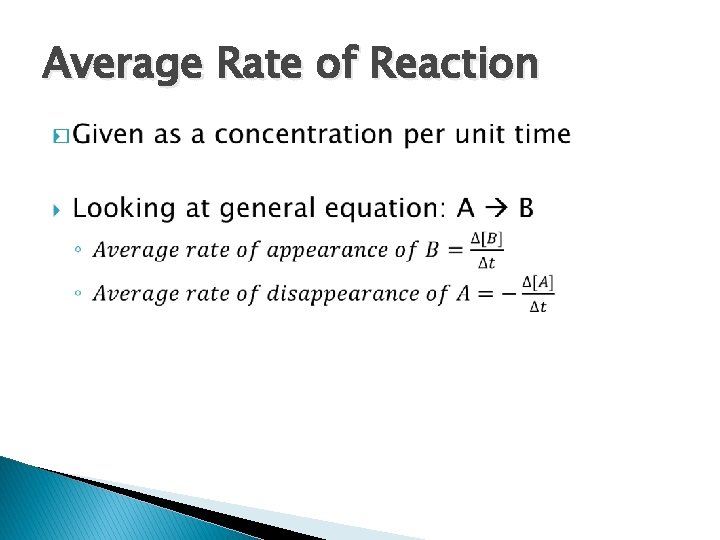
Average Rate of Reaction �
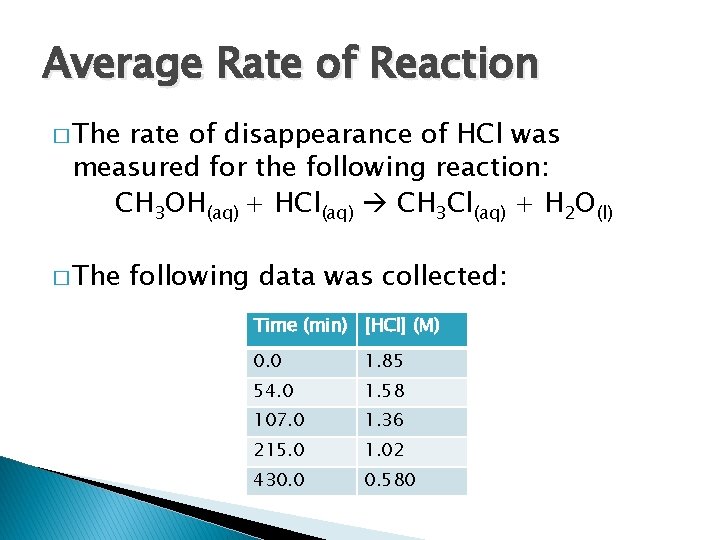
Average Rate of Reaction � The rate of disappearance of HCl was measured for the following reaction: CH 3 OH(aq) + HCl(aq) CH 3 Cl(aq) + H 2 O(l) � The following data was collected: Time (min) [HCl] (M) 0. 0 1. 85 54. 0 1. 58 107. 0 1. 36 215. 0 1. 02 430. 0 0. 580
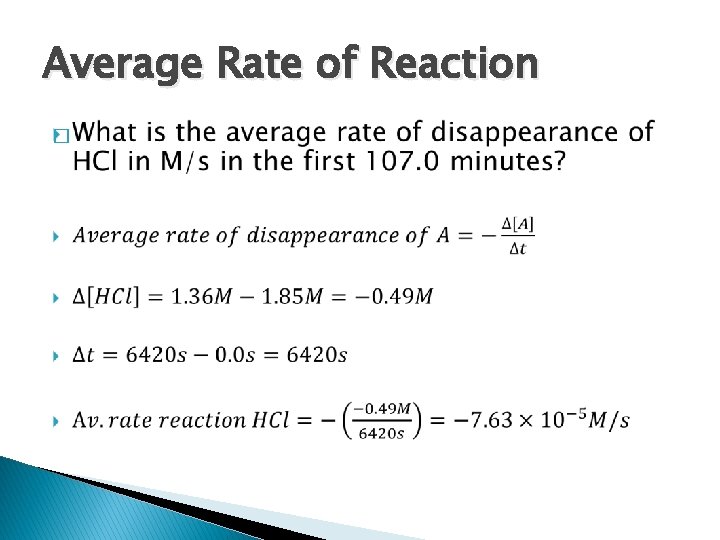
Average Rate of Reaction �
Speeding up Reaction Rate � Solids: ◦ Higher subdivision ◦ Higher concentration of reactants � Gases: ◦ Higher pressure
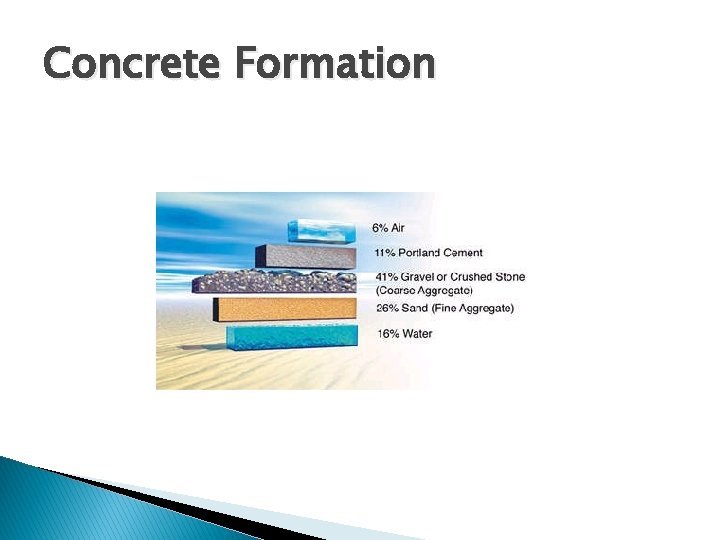
Concrete Formation � Five ◦ ◦ ◦ ingredients: Coarse aggregates Fine aggregates Water Mixtures Portland cement � Concrete formation is an exothermic reaction

Aggregates � Serve as reinforcement to add strength to the concrete 1. Coarse (gravel, crushed stone) 2. Fine (sand)
Mixtures � Accelerating (accelerators) ◦ Added to concrete to reduce setting time ◦ Accelerate early strength (calcium chloride in non steel applications) � Retarding (retarders) ◦ Used in hot weather to delay setting time ◦ Many act as water reducers (sugar)

Mixtures � Fly ash (by-product of coal burning plants) ◦ Can replace 15 -30% of concrete in mixture ◦ Improves workability and reduces heat generated by concrete � Microscopic air bubbles (air entraining) ◦ Used when exposed to freezing/thawing and deicing salts ◦ When hardened concrete freezes the ice inside will expand into the air bubbles instead of cracking concrete
Temperature and Concrete � Cold weather ◦ Concrete sets more slowly ◦ Water expands when frozen, causes cracks if not set before exposure to cold � Hot weather ◦ Top layer solidifies faster than bottom ◦ Concrete shrinks as it solidifies and will cause an uneven product
Concrete Formation

Collision Theory � When particles of reactants collide there are only a certain percentage of collisions that are successful � In successful collision particles must collide at proper orientation and with enough energy to break existing bonds

Collision Theory � Increasing the temperature and concentration of reactants increasing the frequency of collisions as well as the energy in each collision ◦ Increases the likelihood of a successful collision
Activation Energy � Difference between the initial energy of reactants and the transition state ◦ Is the level of energy that must be met in order for a collision to be successful � Transition state is the energy maximum at which the activated complex is formed
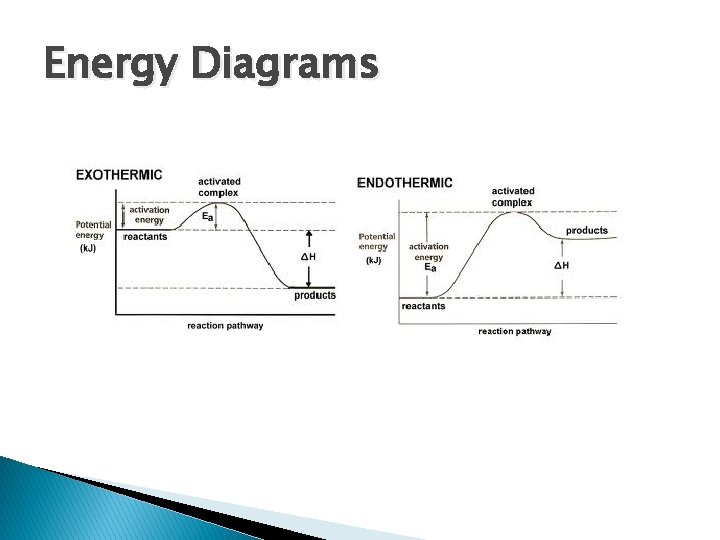
Energy Diagrams
Catalyst � Catalyst is a substance that influences the rate of reaction ◦ Remains unchanged at the end of the reaction � Lower the activation energy of the reaction � Examples: ◦ Enzymes ◦ Platinum/Rhodium
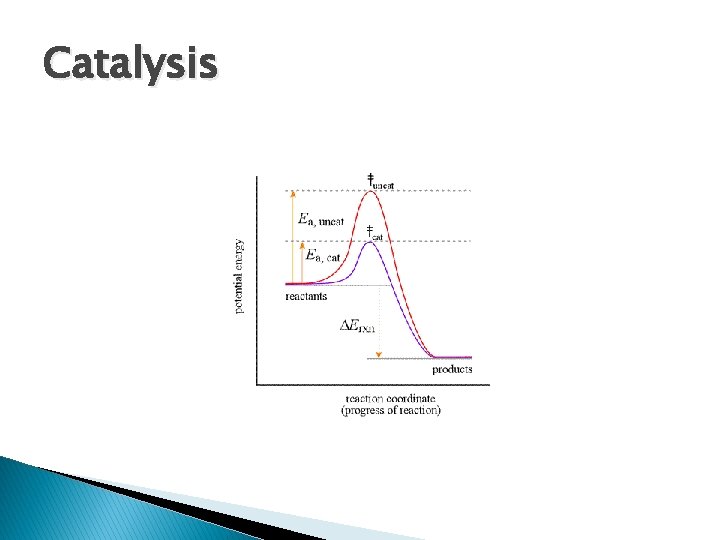
Catalysis
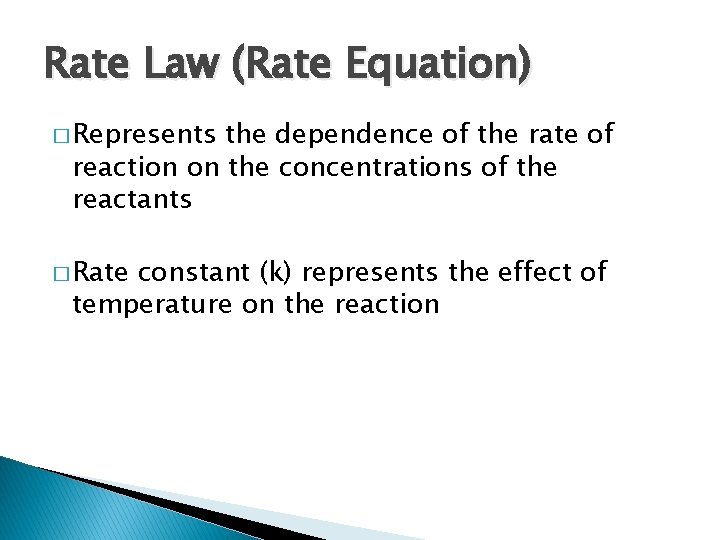
Rate Law (Rate Equation) � Represents the dependence of the rate of reaction on the concentrations of the reactants � Rate constant (k) represents the effect of temperature on the reaction
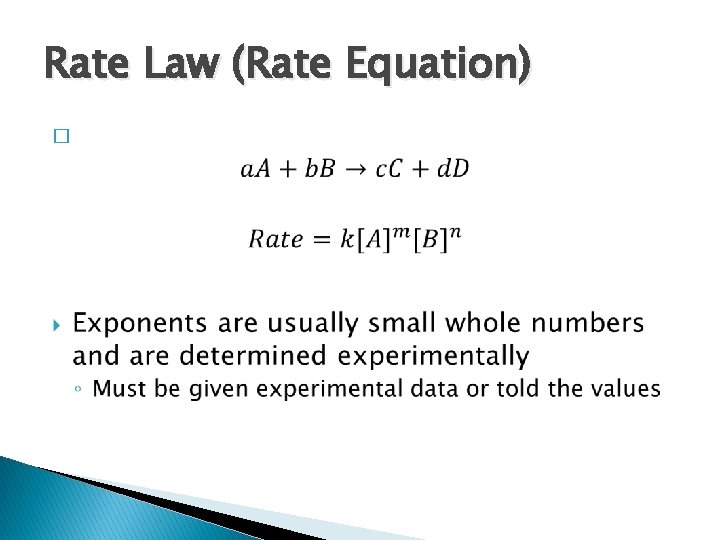
Rate Law (Rate Equation) �
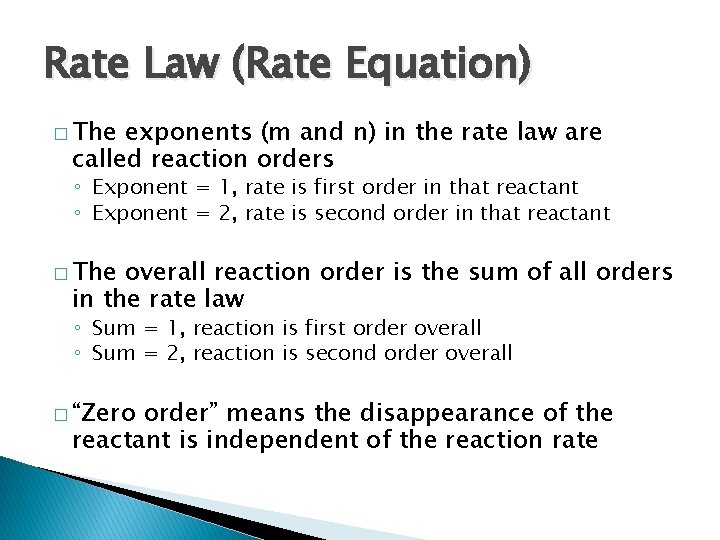
Rate Law (Rate Equation) � The exponents (m and n) in the rate law are called reaction orders ◦ Exponent = 1, rate is first order in that reactant ◦ Exponent = 2, rate is second order in that reactant � The overall reaction order is the sum of all orders in the rate law ◦ Sum = 1, reaction is first order overall ◦ Sum = 2, reaction is second order overall � “Zero order” means the disappearance of the reactant is independent of the reaction rate
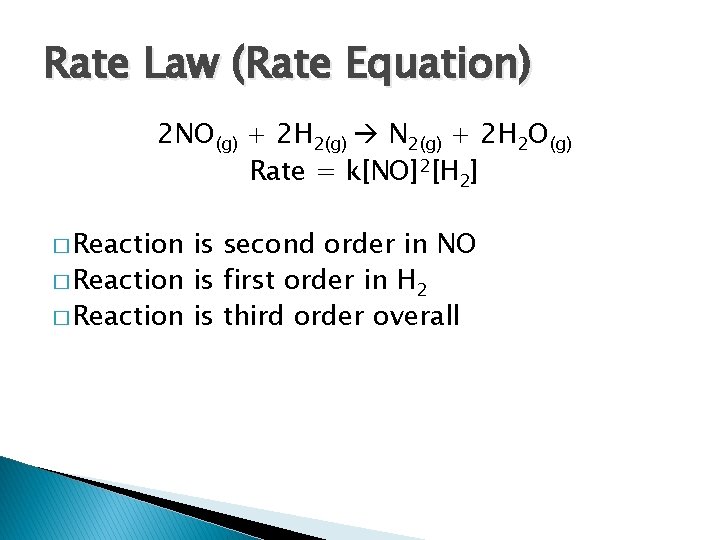
Rate Law (Rate Equation) 2 NO(g) + 2 H 2(g) N 2(g) + 2 H 2 O(g) Rate = k[NO]2[H 2] � Reaction is second order in NO � Reaction is first order in H 2 � Reaction is third order overall
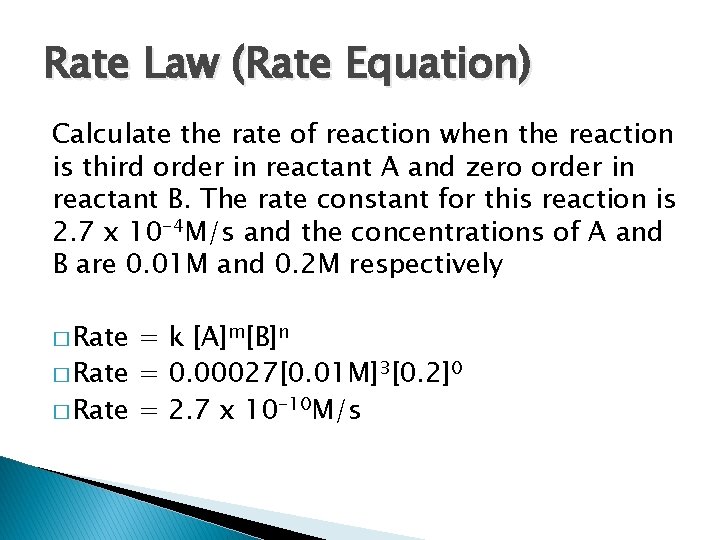
Rate Law (Rate Equation) Calculate the rate of reaction when the reaction is third order in reactant A and zero order in reactant B. The rate constant for this reaction is 2. 7 x 10 -4 M/s and the concentrations of A and B are 0. 01 M and 0. 2 M respectively � Rate = k [A]m[B]n � Rate = 0. 00027[0. 01 M]3[0. 2]0 � Rate = 2. 7 x 10 -10 M/s
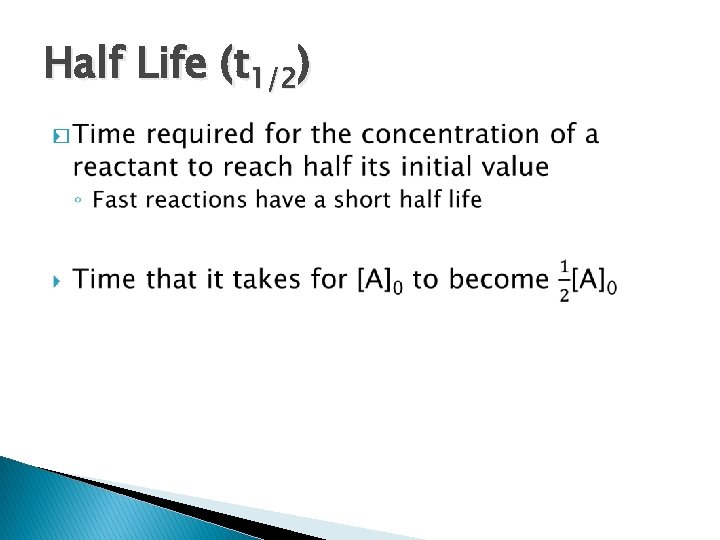
Half Life (t 1/2) �
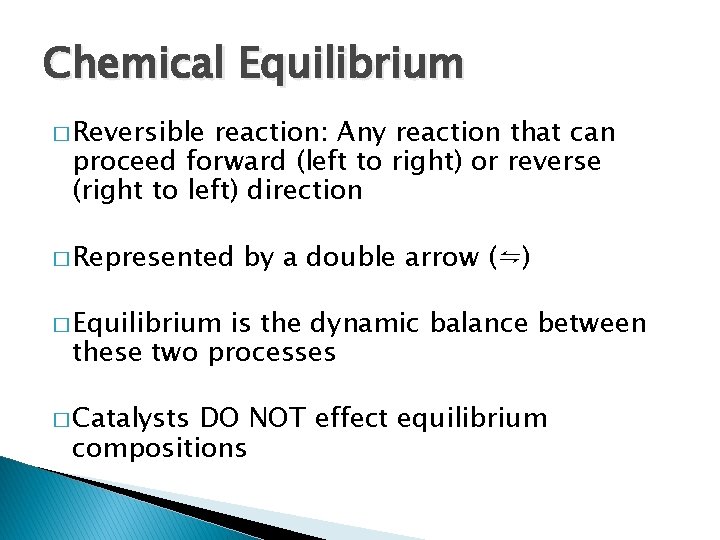
Chemical Equilibrium � Reversible reaction: Any reaction that can proceed forward (left to right) or reverse (right to left) direction � Represented by a double arrow (⇋) � Equilibrium is the dynamic balance between these two processes � Catalysts DO NOT effect equilibrium compositions
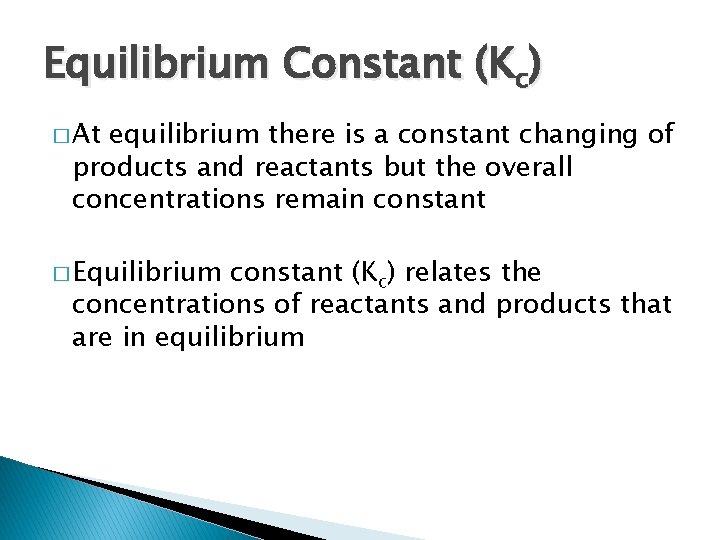
Equilibrium Constant (Kc) � At equilibrium there is a constant changing of products and reactants but the overall concentrations remain constant � Equilibrium constant (Kc) relates the concentrations of reactants and products that are in equilibrium
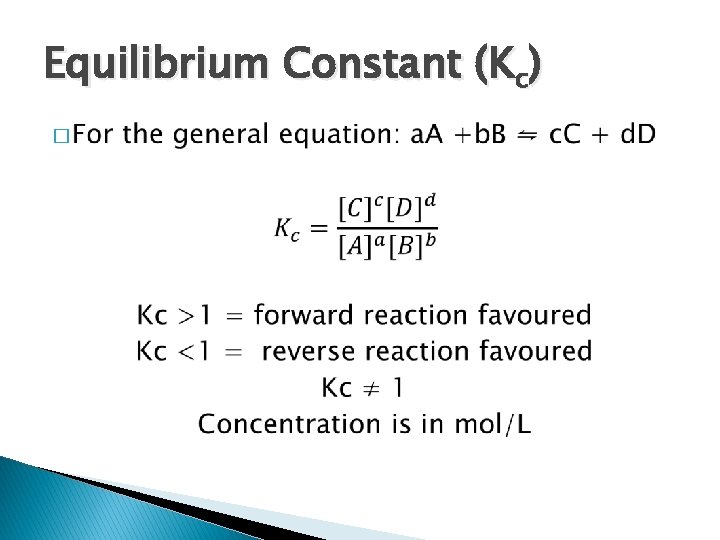
Equilibrium Constant (Kc) �
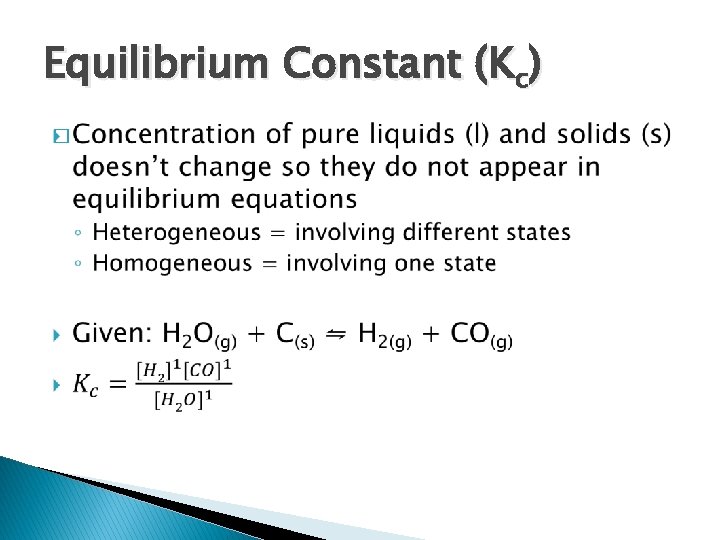
Equilibrium Constant (Kc) �
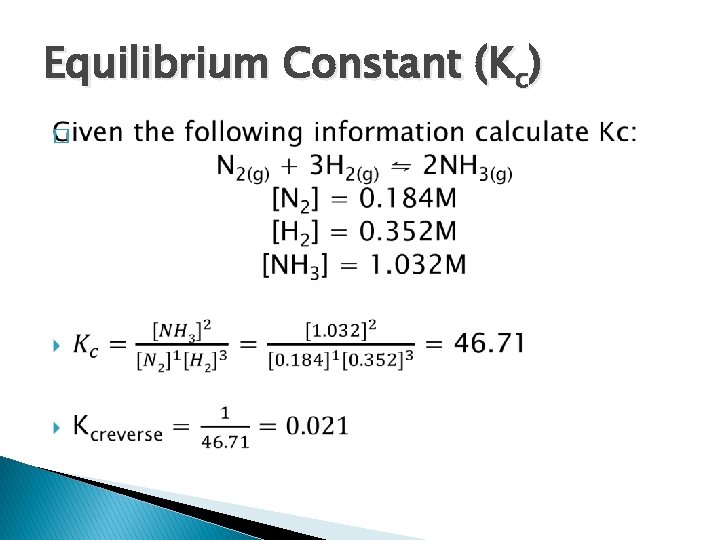
Equilibrium Constant (Kc) �
Equilibrium Constant (Kp) � With gases the pressure (atm) of the reactants and products can be compared ◦ Kc becomes Kp � Kc and Kp vary with temperature
La Châtelier’s Principle � When a system at equilibrium is disturbed it will compensate for the disturbance � Examples ◦ ◦ of altering: Addition of Catalyst (Trick! No change!) Concentrations Pressures Temperature
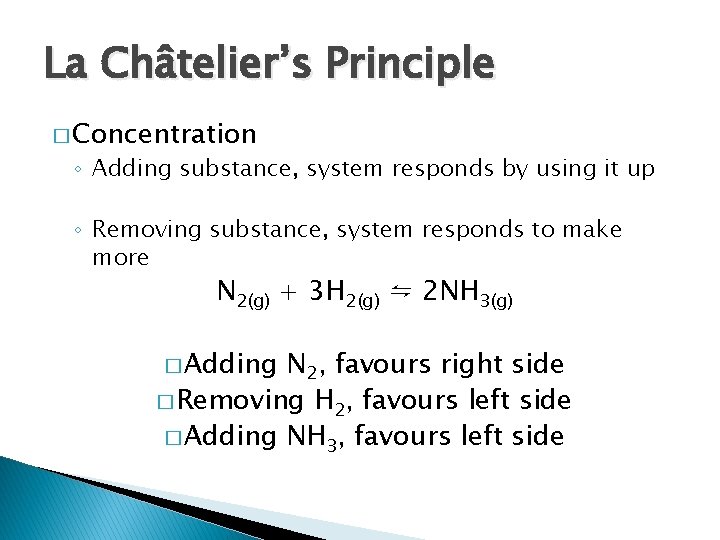
La Châtelier’s Principle � Concentration ◦ Adding substance, system responds by using it up ◦ Removing substance, system responds to make more N 2(g) + 3 H 2(g) ⇋ 2 NH 3(g) � Adding N 2, favours right side � Removing H 2, favours left side � Adding NH 3, favours left side
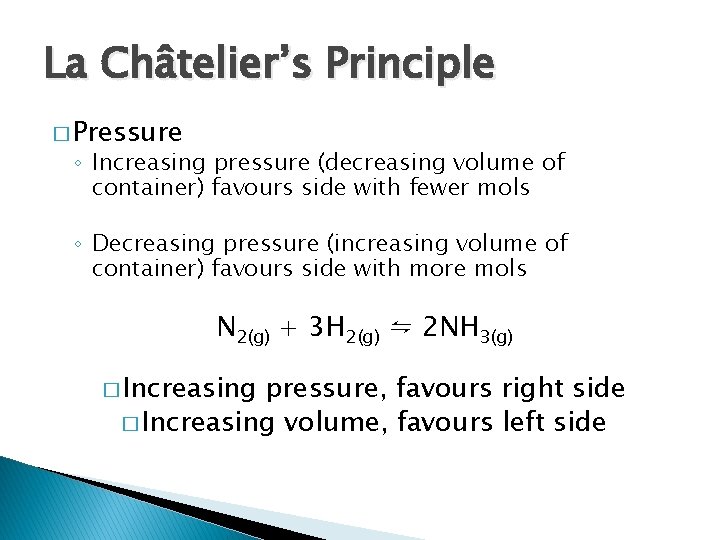
La Châtelier’s Principle � Pressure ◦ Increasing pressure (decreasing volume of container) favours side with fewer mols ◦ Decreasing pressure (increasing volume of container) favours side with more mols N 2(g) + 3 H 2(g) ⇋ 2 NH 3(g) � Increasing pressure, favours right side � Increasing volume, favours left side
La Châtelier’s Principle � Temperature ◦ Heat acts as a product or reactant �Exothermic reactions, heat = product �Endothermic reactions, heat = reactant ◦ When temperature of system is increased �Adding reactant to endothermic reaction �Adding product to exothermic reaction ◦ When temperature of system is decreased �Adding product to endothermic reaction �Adding reactant to exothermic reaction
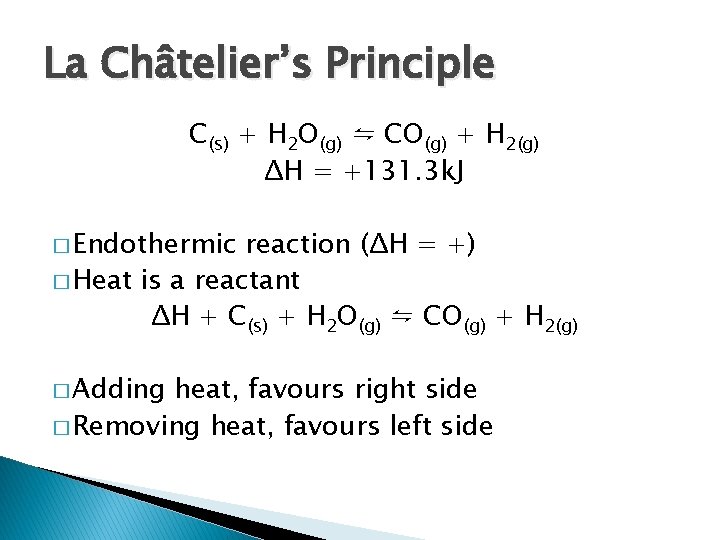
La Châtelier’s Principle C(s) + H 2 O(g) ⇋ CO(g) + H 2(g) ΔH = +131. 3 k. J � Endothermic reaction (ΔH = +) � Heat is a reactant ΔH + C(s) + H 2 O(g) ⇋ CO(g) + H 2(g) � Adding heat, favours right side � Removing heat, favours left side
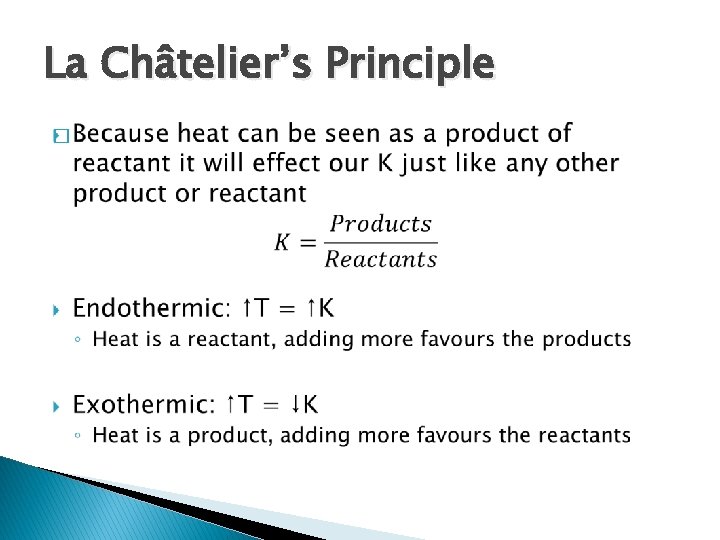
La Châtelier’s Principle �
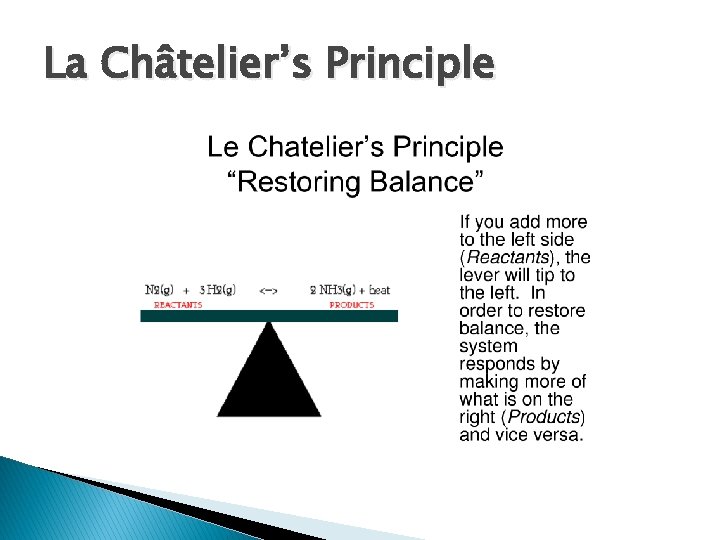
La Châtelier’s Principle
- Slides: 37