Unit 3 Part 3 Periodic Trends Outline Development










- Slides: 30

Unit 3 Part 3: Periodic Trends

Outline �Development of the Periodic Table �Parts of the Modern Periodic Table �Representative elements, transition elements �Metals, nonmetals and metalloids �Classification of elements using electron configuration �Valence electrons and period �S, p, d, f blocks �Periodic Trends �Atomic radius, ionic radius �Ionization energy �Electronegativity

Development of the Periodic Table �Newlands �Meyer and Mendeleev �Moseley

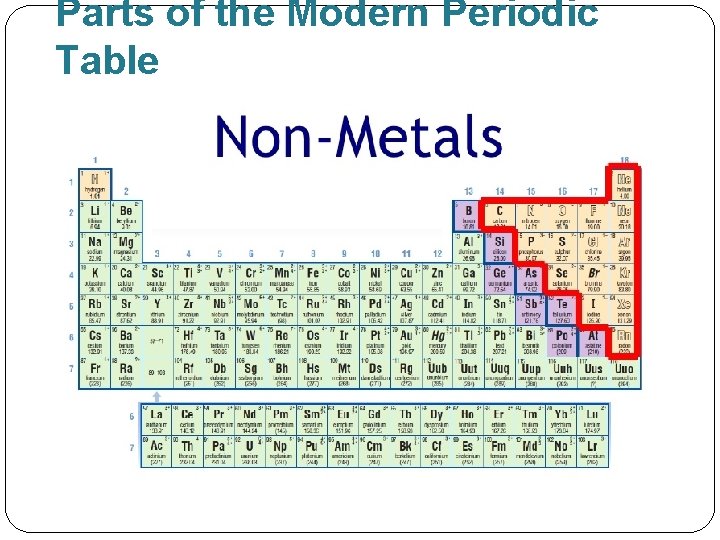
Parts of the Modern Periodic Table �Representative elements: groups 1 -2 and 13 -18 �Transition elements: groups 3 -12 �Elements are classified as metals, metalloids and nonmetals.

Parts of the Modern Periodic Table Metals: �Shiny �Good conductors of electricity and heat �Malleable: can be shaped into many shapes �Ductile: can be drawn into wires

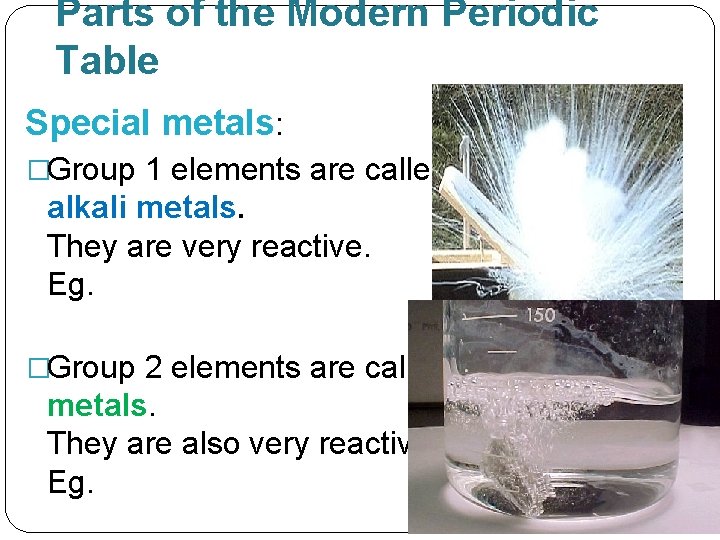
Parts of the Modern Periodic Table Special metals: �Group 1 elements are called alkali metals. They are very reactive. Eg. �Group 2 elements are called alkaline earth metals. They are also very reactive. Eg.

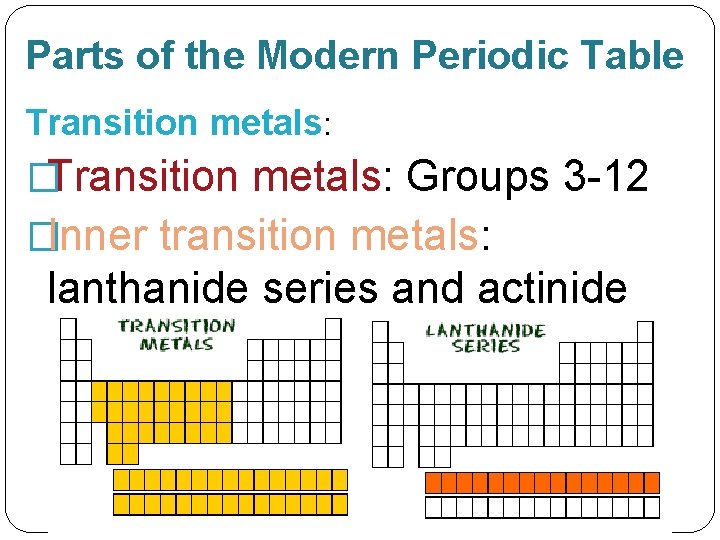
Parts of the Modern Periodic Table Transition metals: �Transition metals: Groups 3 -12 �Inner transition metals: lanthanide series and actinide series.

Parts of the Modern Periodic Table Nonmetals: �Gas, solid, or liquid. �Brittle �Poor conductors of heat and electricity �Eg. Group 17: Halogens �Eg. Group 18: Noble gases

Parts of the Modern Periodic Table

Parts of the Modern Periodic Table Metalloids: �Properties of both metals and non metals �Eg. Silicon, Si:

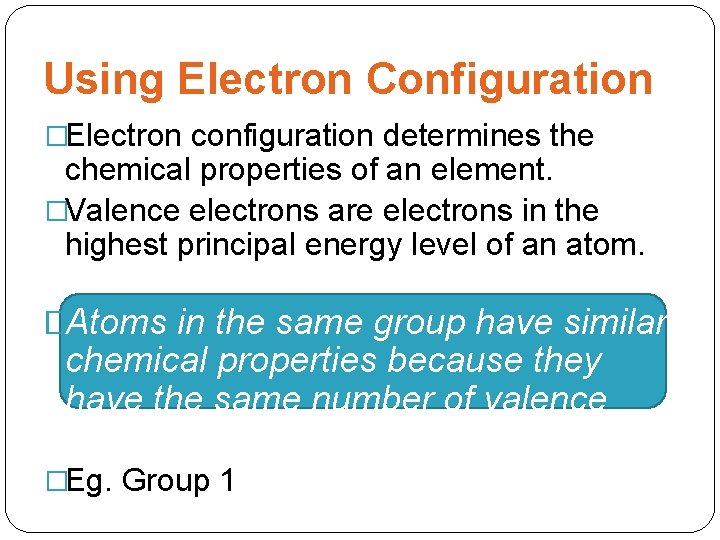
Using Electron Configuration �Electron configuration determines the chemical properties of an element. �Valence electrons are electrons in the highest principal energy level of an atom. �Atoms in the same group have similar chemical properties because they have the same number of valence electrons. �Eg. Group 1

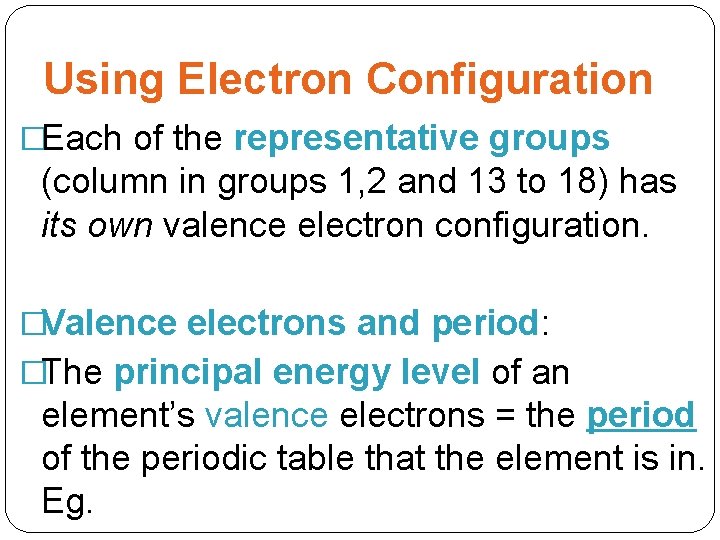
Using Electron Configuration �Each of the representative groups (column in groups 1, 2 and 13 to 18) has its own valence electron configuration. �Valence electrons and period: �The principal energy level of an element’s valence electrons = the period of the periodic table that the element is in. Eg.

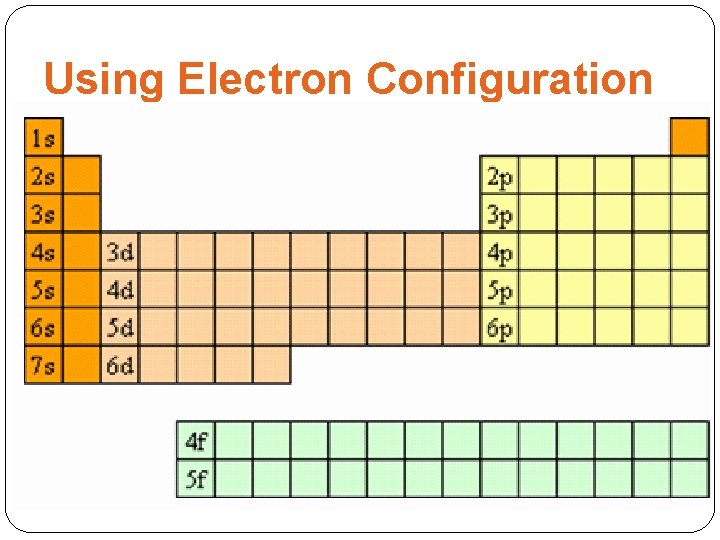
Using Electron Configuration �s-block, p-block, d-block, and f-block elements �The periodic table has columns and rows of varying sizes. �This is because the table has been divided into sections, or blocks, representing the valence electrons’ sublevels. (ie. s, p, d, or f)

Using Electron Configuration � 1. Which groups are the s-block elements? What do the valence electrons for these groups look like? Why are there only two groups in the s-block? � 2. Which groups are the p-block elements? Why are there no p-block elements in period 1? Why are there six groups in the p-block? � 3. What is a characteristic of the d-block? Example:

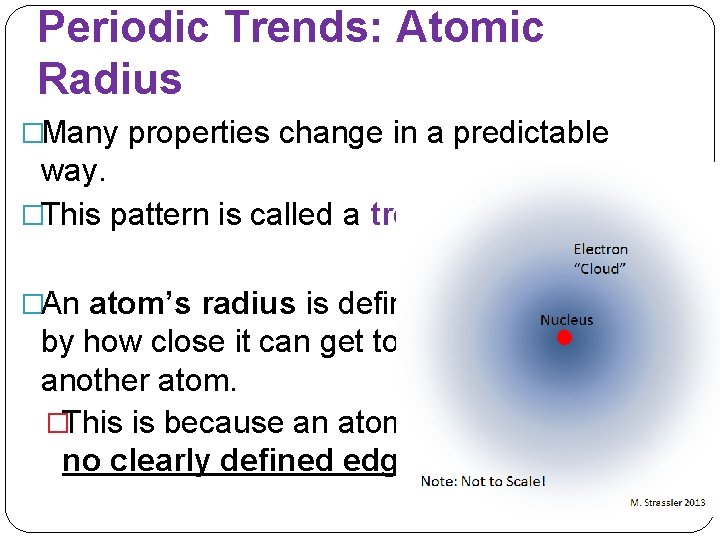
Periodic Trends: Atomic Radius �Many properties change in a predictable way. �This pattern is called a trend. �An atom’s radius is defined by how close it can get to another atom. �This is because an atom has no clearly defined edge.

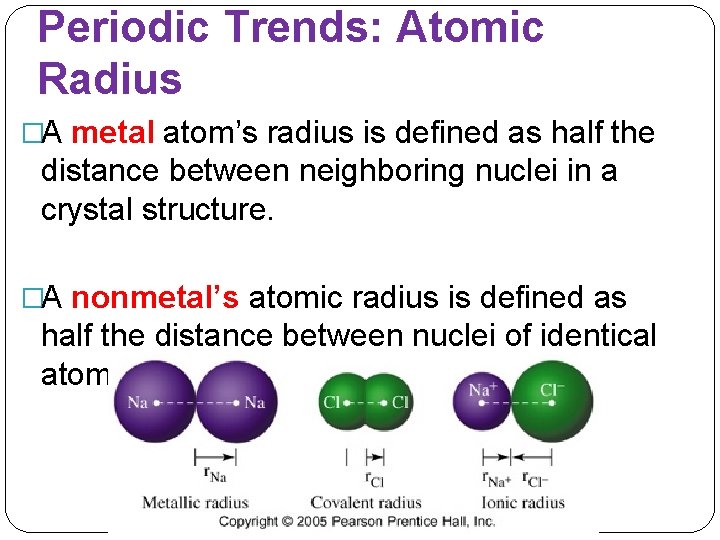
Periodic Trends: Atomic Radius �A metal atom’s radius is defined as half the distance between neighboring nuclei in a crystal structure. �A nonmetal’s atomic radius is defined as half the distance between nuclei of identical atoms that are bonded together.

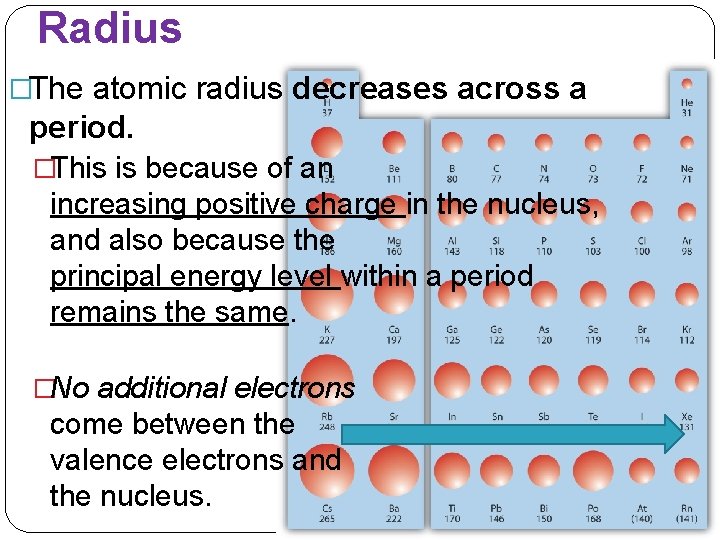
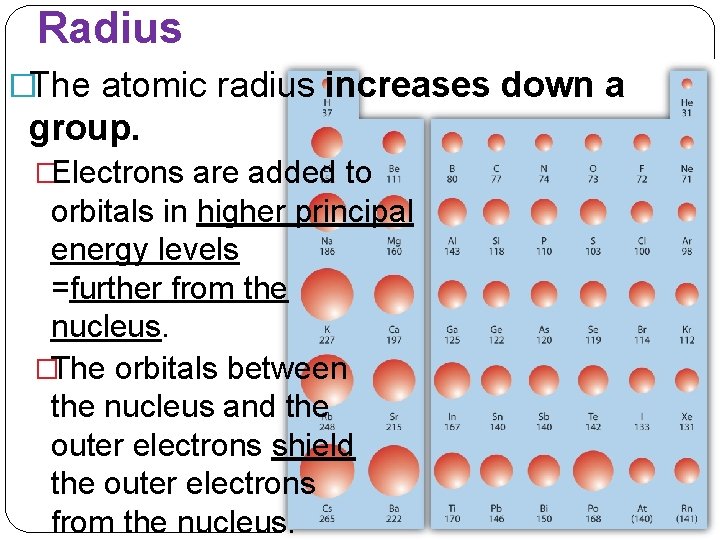
Radius �The atomic radius decreases across a period. �This is because of an increasing positive charge in the nucleus, and also because the principal energy level within a period remains the same. �No additional electrons come between the valence electrons and the nucleus.

Radius �The atomic radius increases down a group. �Electrons are added to orbitals in higher principal energy levels =further from the nucleus. �The orbitals between the nucleus and the outer electrons shield the outer electrons from the nucleus.

Periodic Trends: Atomic Radius �P. 189 Q 16 -19

Periodic Trends: Ionic Radius �Atoms can gain or lose electrons to form a charged version of itself, which is called an ion. �When atoms lose electrons, they form ________ ions called cations. �When atoms gaim electrons, they from ________ ions called anions.

Periodic Trends: Ionic Radius �Atoms can gain or lose electrons to acquire a full set of eight valence electrons. �This is called the octet rule. �This is because the electron configuration of filled s and p orbitals of the same energy level is very stable. �Exception: period 1 elements. They

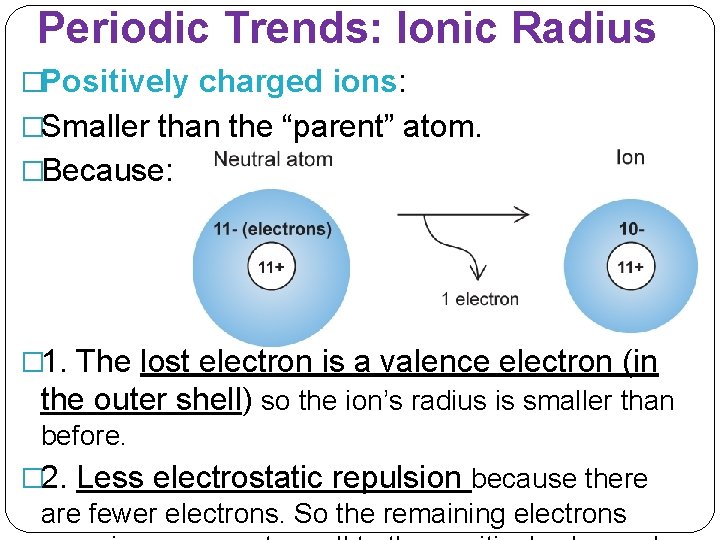
Periodic Trends: Ionic Radius �Positively charged ions: �Smaller than the “parent” atom. �Because: � 1. The lost electron is a valence electron (in the outer shell) so the ion’s radius is smaller than before. � 2. Less electrostatic repulsion because there are fewer electrons. So the remaining electrons

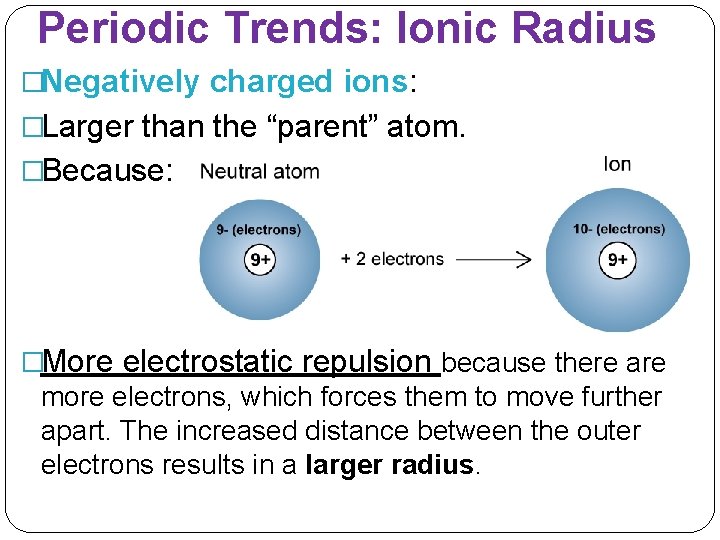
Periodic Trends: Ionic Radius �Negatively charged ions: �Larger than the “parent” atom. �Because: �More electrostatic repulsion because there are more electrons, which forces them to move further apart. The increased distance between the outer electrons results in a larger radius.

Periodic Trends: Ionic Radius

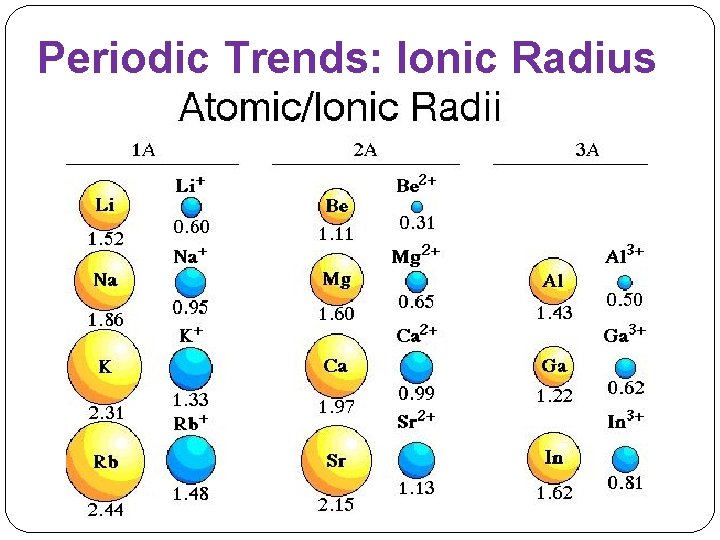
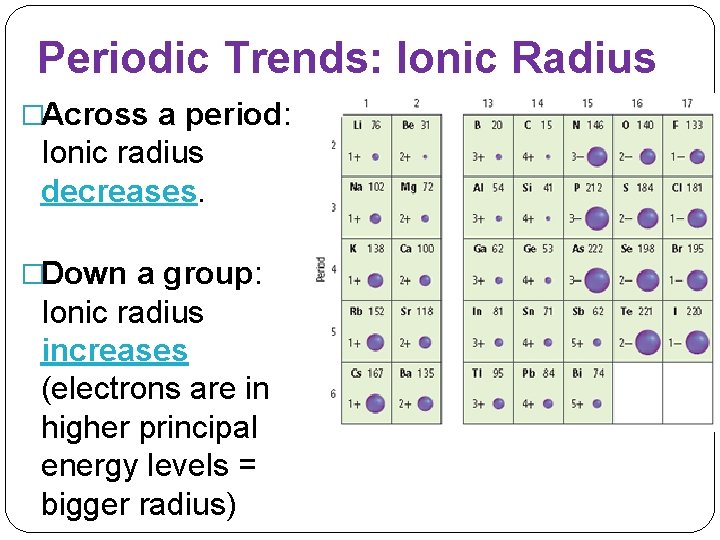
Periodic Trends: Ionic Radius �Across a period: Ionic radius decreases. �Down a group: Ionic radius increases (electrons are in higher principal energy levels = bigger radius)

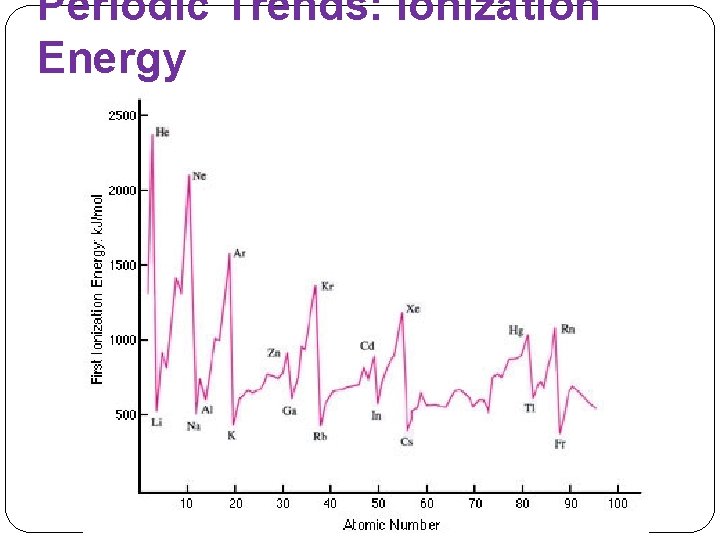
Periodic Trends: Ionization Energy �Energy required to remove an electron from a gaseous atom. �First ionization energy: �Second ionization energy: �High ionization energy means _____

Periodic Trends: Ionization Energy

Periodic Trends: Ionization Energy �Across a period: ionization energies usually increase. �Down a group: ionization energies usually decrease. �Jump in ionization energies after all the valence electrons are removed.

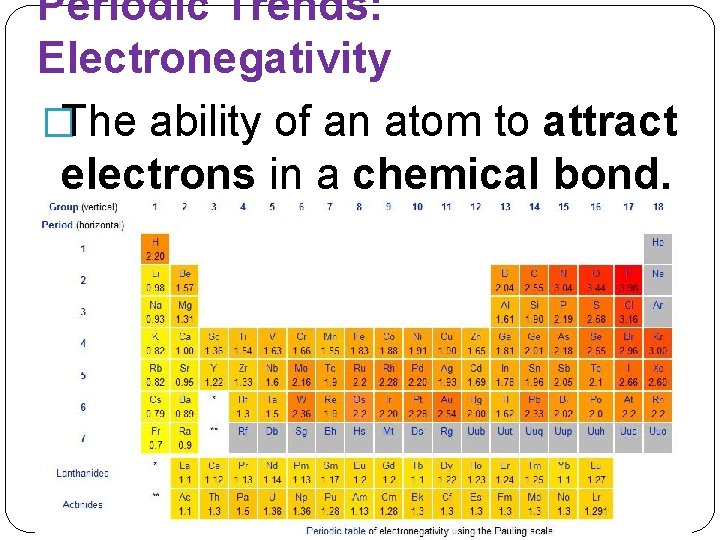
Periodic Trends: Electronegativity �The ability of an atom to attract electrons in a chemical bond.

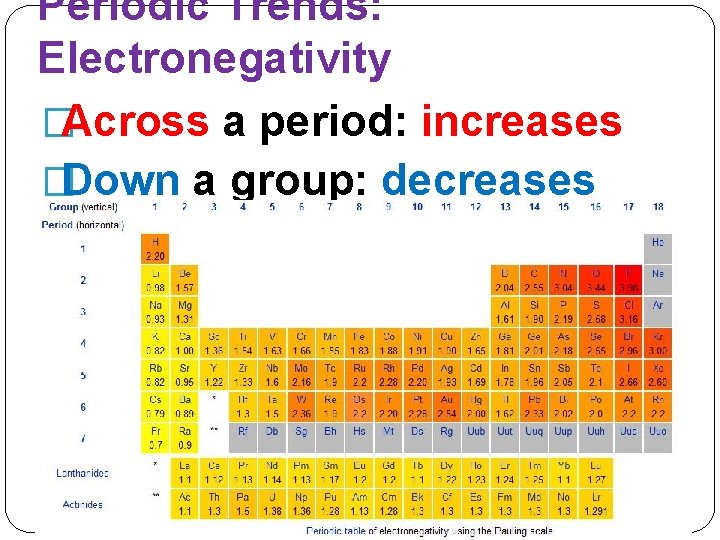
Periodic Trends: Electronegativity �Across a period: increases �Down a group: decreases