Unit 3 Modern Atomic Theory the Periodic Table
Unit 3 – Modern Atomic Theory & the Periodic Table The Periodic Table I. History I II III

Mendeleev z Dmitri Mendeleev (1869, Russian) y Organized elements by increasing atomic mass. y Elements with similar properties were grouped together. y There were some discrepancies.

Dmitri Mendeléev

Mendeleev z Dmitri Mendeleev (1869, Russian) y Predicted properties of undiscovered elements.

Moseley z Henry Moseley (1913, British) y Organized elements by increasing atomic number. y Resolved discrepancies in Mendeleev’s arrangement.

Unit 3 – Modern Atomic Theory & the Periodic Table The Periodic Table II. Organization of the Elements I II III

Periods z Elements are arranged in seven horizontal rows, in order of increasing atomic number from left to right and from top to bottom. y Rows are called periods and are numbered from 1 to 7. x Represent principal energy levels (n)

Groups z Elements with similar chemical properties form vertical columns, called groups (families). z Groups 1 A - 8 A are the representative elements. y s and p-blocks z The B groups are the transition elements.
Inner Transition Elements z The two rows of 14 elements at the bottom of the periodic table are the inner transition elements. y Lanthanide Series - the 4 f row that includes # 57 (Lanthanum) - #71 Lu y Actinide Series - the 5 f row that includes #89 Ac (Actinium) - #102 No
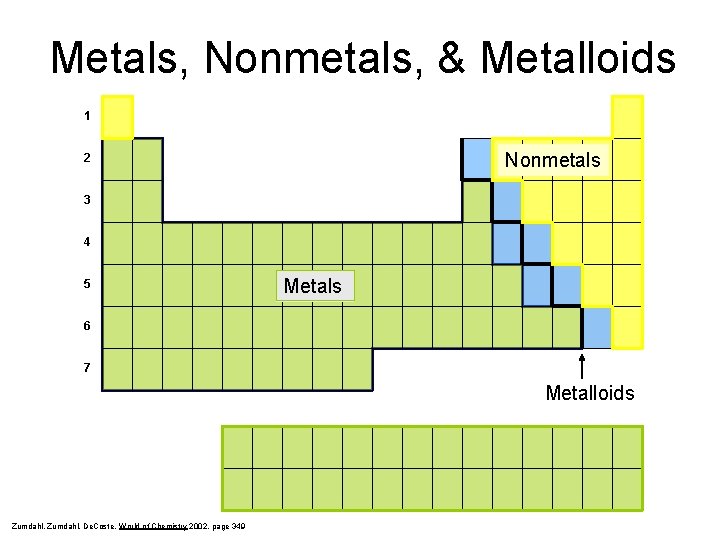
Metals, Nonmetals, & Metalloids 1 Nonmetals 2 3 4 5 Metals 6 7 Metalloids Zumdahl, De. Coste, World of Chemistry 2002, page 349

Metals z To the left of the staircase (or below) z Most are solids except for Hg which is a liquid z Malleable, lustrous, ductile, good conductors of heat & electricity

Nonmetals z To the right of the staircase (or above) z Gases or brittle solids at room temperature z Poor conductors of heat & electricity (insulators)

Metalloids z Semi-metals y These elements border the staircase and have properties of both metals and nonmetals. z Include the following elements: B, Si, Ge, As, Sb, Te, and At z Dull, brittle, semi-conductors (used in computer chips)
Alkali Metals (Group 1 A) z very reactive z good conductors z end in s 1 z need to lose 1 e- to have noble gas configuration z reactivity increases as you go down the group
Alkaline Earth Metals (Group 2 A) z less reactive than alkali metals z end in s 2 z need to lose 2 e- to have noble gas configuration
Halogens (Group 7 A) z combine easily with alkali metals z exist as diatomic molecules y F 2, Cl 2, etc. z electron configuration ends in p 5 z need to gain 1 e- to achieve noble gas configuration
Noble Gases (Group 8 A) z full outer energy level z electron configuration ends in p 6 z do not form chemical compounds easily y also called inert gases
Unit 3 – Modern Atomic Theory & the Periodic Table The Periodic Table III. Periodic Trends I II III
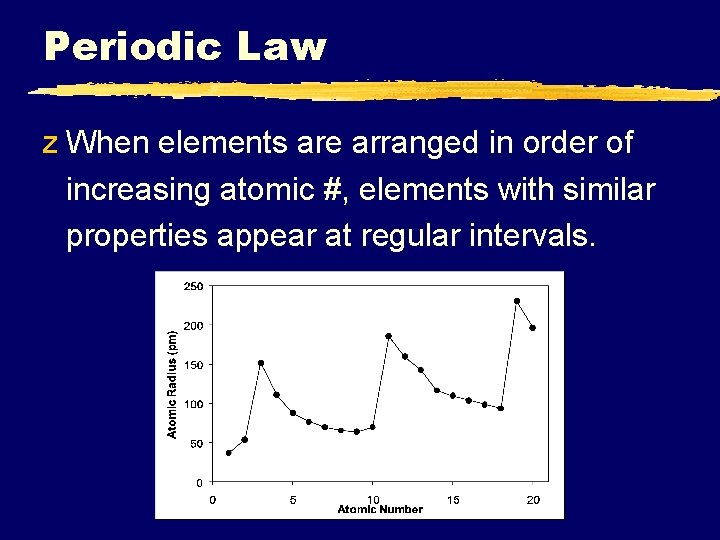
Periodic Law z When elements are arranged in order of increasing atomic #, elements with similar properties appear at regular intervals.

Periodic Properties z Atomic Radius y Size of atom z Ionization Energy y Energy required to remove an e- from a neutral atom. z Electronegativity y Ability of an atom to attract electrons to itself in a chemical bond
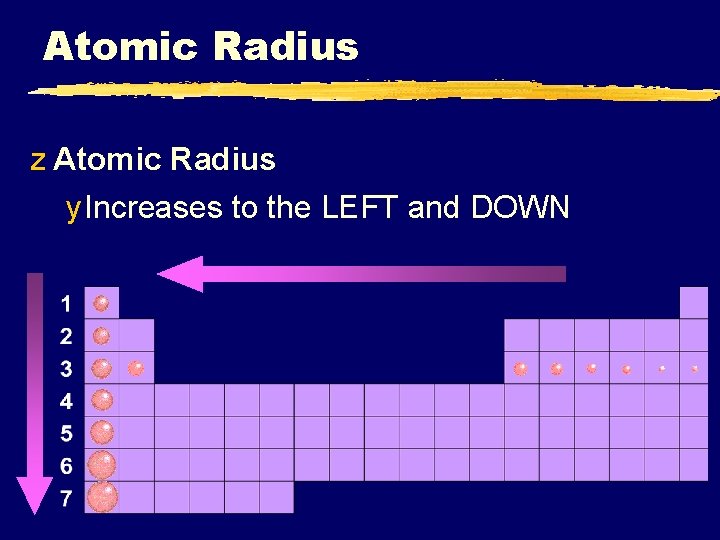
Atomic Radius z Atomic Radius y Increases to the LEFT and DOWN

Atomic Radius z Why larger going down? y Higher energy levels have larger orbitals y Shielding - core e- block the attraction between the nucleus and the valence ez Why smaller to the right? y Increased nuclear charge without additional shielding pulls e- in tighter
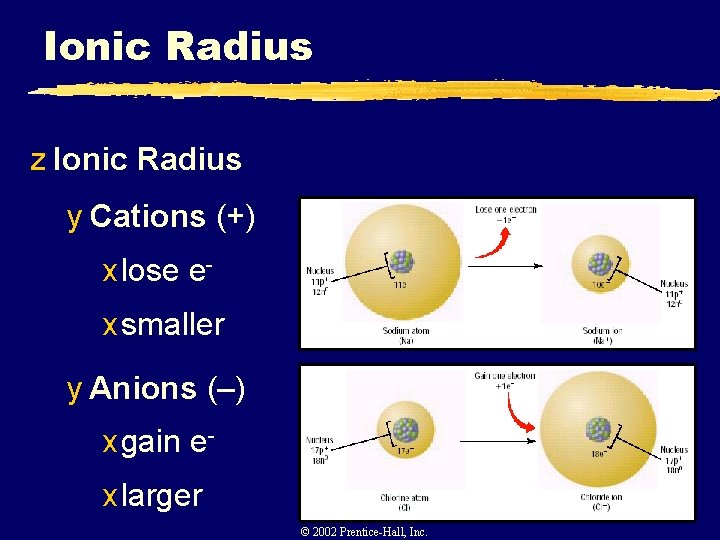
Ionic Radius z Ionic Radius y Cations (+) x lose ex smaller y Anions (–) x gain ex larger © 2002 Prentice-Hall, Inc.
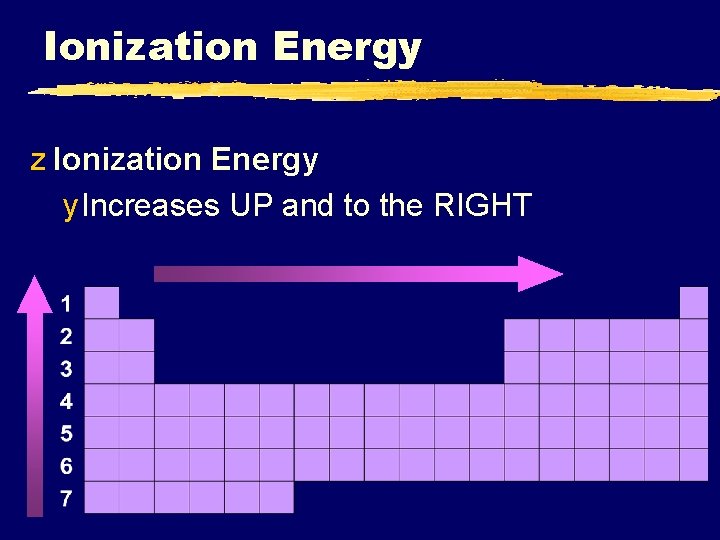
Ionization Energy z Ionization Energy y Increases UP and to the RIGHT
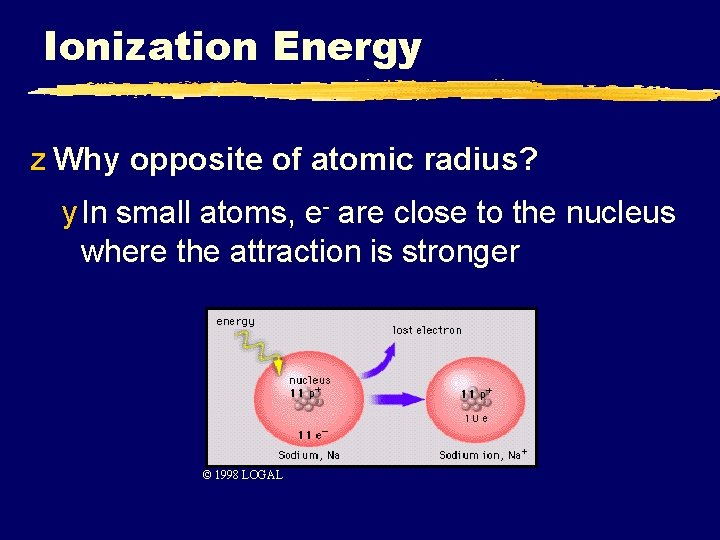
Ionization Energy z Why opposite of atomic radius? y In small atoms, e- are close to the nucleus where the attraction is stronger © 1998 LOGAL
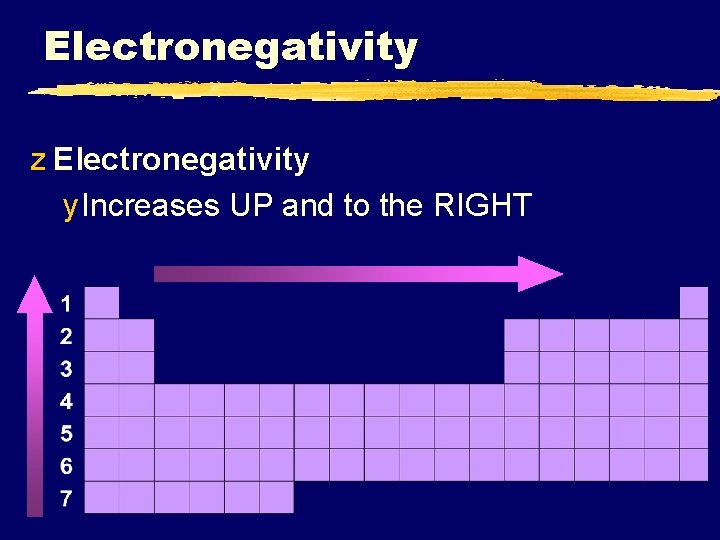
Electronegativity z Electronegativity y Increases UP and to the RIGHT

Electronegativity z Why larger going across? y Stronger tendency to attract electrons and form negative ions z Why smaller going down a group? y Shielding - core e- block the attraction between the nucleus and the valence e-

Examples z Which atom has the larger radius? y. Be or Ba Ba y. Ca or Br Ca
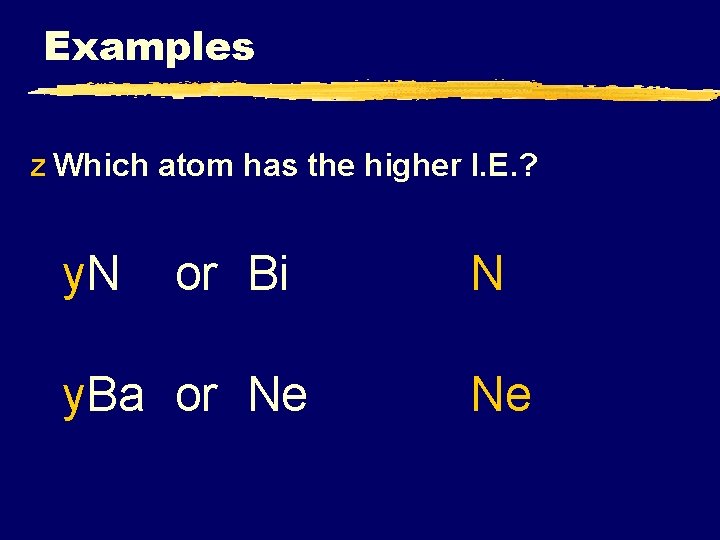
Examples z Which atom has the higher I. E. ? y. N or Bi y. Ba or Ne N Ne

Examples z Which atom has higher electronegativity? y. Li or C C y. Cr or Br Br

Examples z Which particle has the larger radius? y. S or S 2 - y. Al or Al 3+ S 2 Al

The END of Unit 3!
- Slides: 32