Unit 3 Matter as Solutions Acids and Bases

Unit 3 Matter as Solutions, Acids, and Bases Chapter 5 Solutions Section 5. 1 Classifying Solutions (P. 166– 175)

First some definitions: • Solution – is a homogeneous mixture of a solid, liquid or gaseous solute in a solid, liquid or gaseous solvent.

• Alloys – a solid solution of metals. • Amalgams – solutions of mercury in other metals. • Aqueous Solutions – solutions in which the solvent is water.
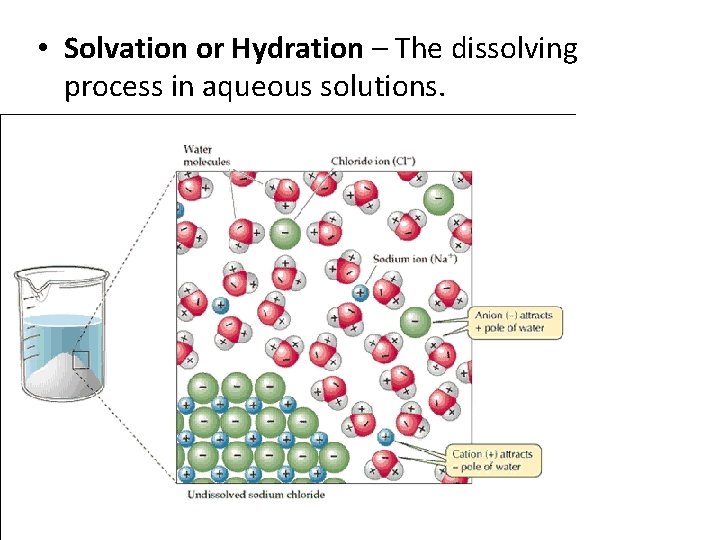
• Solvation or Hydration – The dissolving process in aqueous solutions.

• Colloids – appear to be solutions but are actually heterogeneous mixtures, but the clumps of particles are too small to see with the unaided eye. Ex. milk, fog, mayonnaise, marshmallows, smoke, and shaving cream.

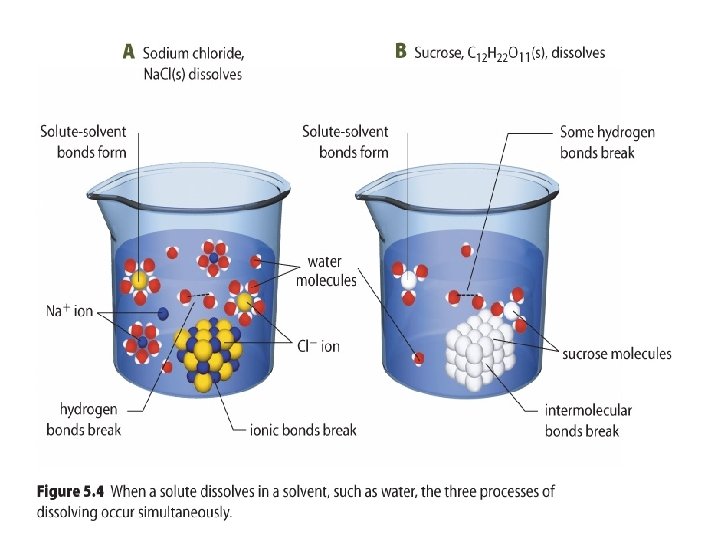
Dissolving and Forces of Attraction • Dissolving is a physical change, not a chemical change. No chemical reaction has occurred.

When a solute dissolves three processes occur simultaneously: • The bonds holding the molecules or ions together are broken. This process requires energy (endothermic). • Some of the intermolecular forces between the particles of the solvent also break (endothermic). • The molecules or ions of the solute become attracted by the molecules of the solvent and new bonds are formed. This process releases energy (exothermic). The solute particles are dispersed evenly throughout the solution.
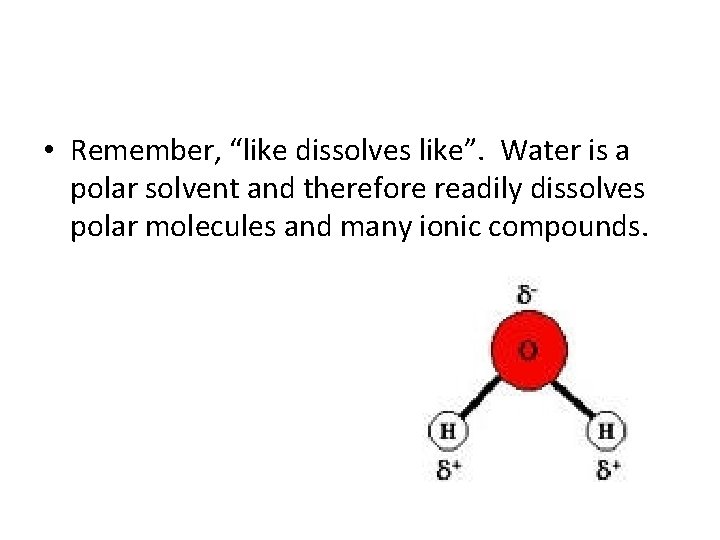
• Remember, “like dissolves like”. Water is a polar solvent and therefore readily dissolves polar molecules and many ionic compounds.
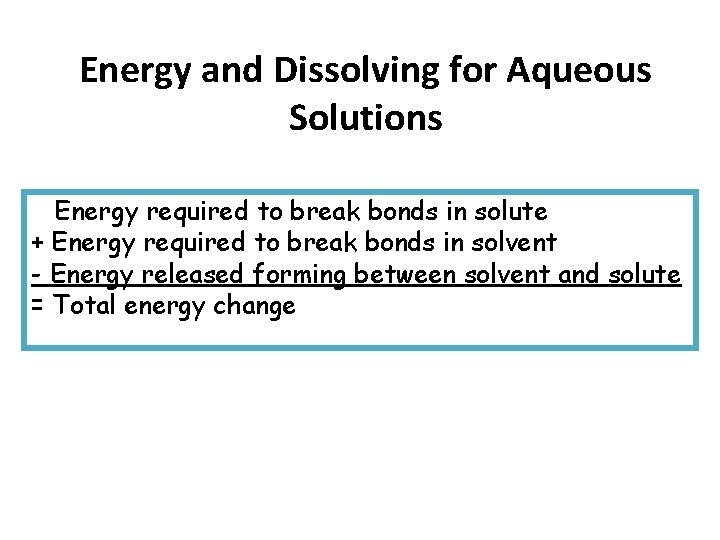
Energy and Dissolving for Aqueous Solutions Energy required to break bonds in solute + Energy required to break bonds in solvent - Energy released forming between solvent and solute = Total energy change

• If more energy is required to break the bonds than is produced when forming new bonds, the process is endothermic. Ex. solvation of NH 4 NO 3 (s) in cold packs. • If more energy is released by the formation of new bonds than is required to break the bonds, the process is exothermic. Ex. solvation of Na. OH (s)
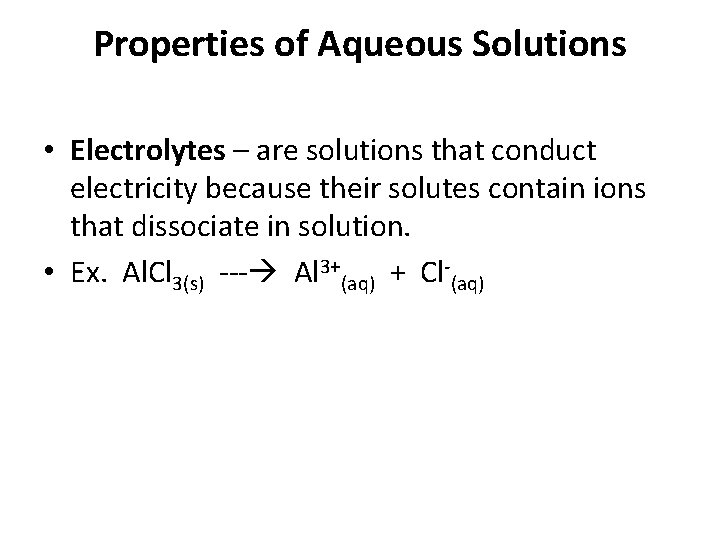
Properties of Aqueous Solutions • Electrolytes – are solutions that conduct electricity because their solutes contain ions that dissociate in solution. • Ex. Al. Cl 3(s) Al 3+(aq) + Cl (aq)

Your body requires electrolytes like sodium, potassium, and calcium ions for muscle contraction, nerve conduction, waste removal, maintenance of blood pressure, and may other important functions. If your body looses a lot of fluid in a short period of time, through sweating for example, the balance of electrolytes is upset. Sport drinks are designed to replace both water and important electrolytes as well as provide glucose as an energy source.
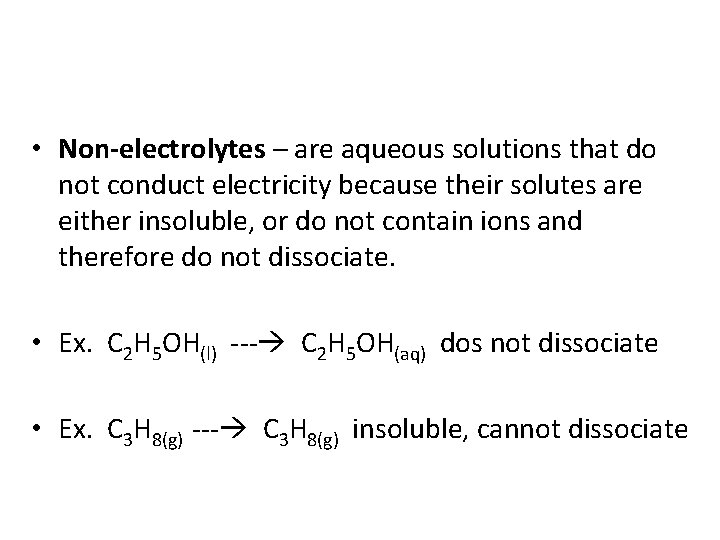
• Non-electrolytes – are aqueous solutions that do not conduct electricity because their solutes are either insoluble, or do not contain ions and therefore do not dissociate. • Ex. C 2 H 5 OH(l) C 2 H 5 OH(aq) dos not dissociate • Ex. C 3 H 8(g) insoluble, cannot dissociate

Ionic Compounds in Solution • Ionic compounds dissociate into charged particles when dissolved. Therefore, ionic compounds dissolved in water form electrolytes. • Ionic bonds are broken when ionic solutes are dissolved.
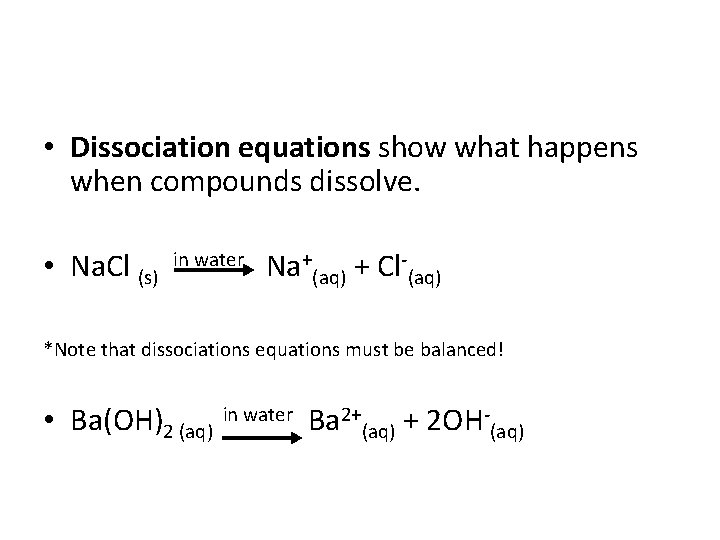
• Dissociation equations show what happens when compounds dissolve. • Na. Cl (s) in water Na+(aq) + Cl (aq) *Note that dissociations equations must be balanced! • Ba(OH)2 (aq) in water Ba 2+(aq) + 2 OH (aq)

• Some ionic compounds do not dissolve appreciably in water and are said to be insoluble or (slightly soluble). Insoluble ionic compounds do not dissociate and therefore do not form electrolytes. • In actual fact all ionic compounds dissolve in water to some degree. However, for some compounds the amount of compound that will actually go into solution is so small that it can be ignored and the compound considered insoluble.
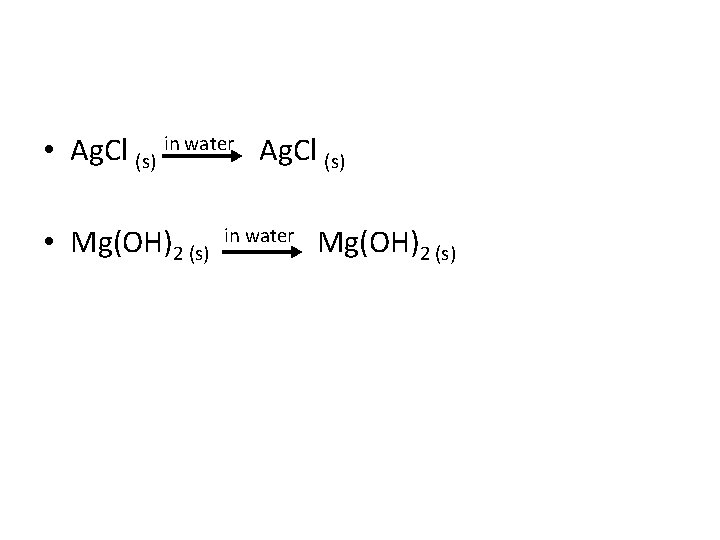
• Ag. Cl (s) in water Ag. Cl (s) • Mg(OH)2 (s) in water Mg(OH)2 (s)

• Use the solubility chart or your periodic table to determine if these ionic compounds will dissociate. Use the appropriate subscript to indicate solubility. • Na. NO 3 ( ) and Cu. SO 4 ( )
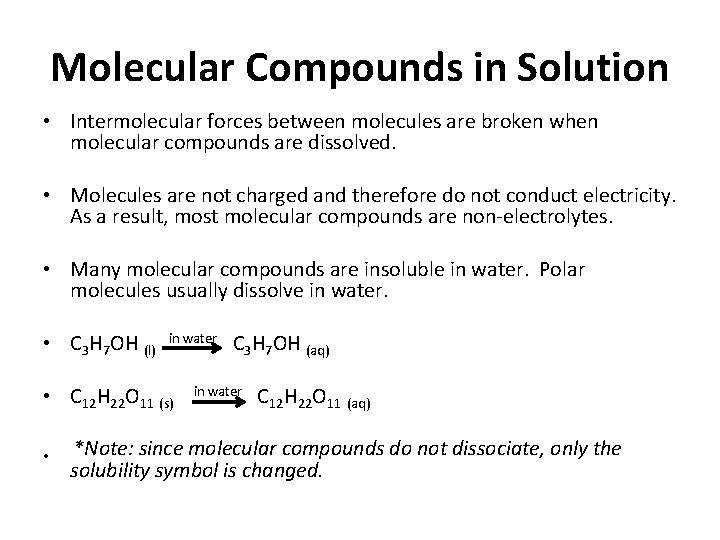
Molecular Compounds in Solution • Intermolecular forces between molecules are broken when molecular compounds are dissolved. • Molecules are not charged and therefore do not conduct electricity. As a result, most molecular compounds are non electrolytes. • Many molecular compounds are insoluble in water. Polar molecules usually dissolve in water. • C 3 H 7 OH (l) in water C 3 H 7 OH (aq) • C 12 H 22 O 11 (s) • in water C 12 H 22 O 11 (aq) *Note: since molecular compounds do not dissociate, only the solubility symbol is changed.
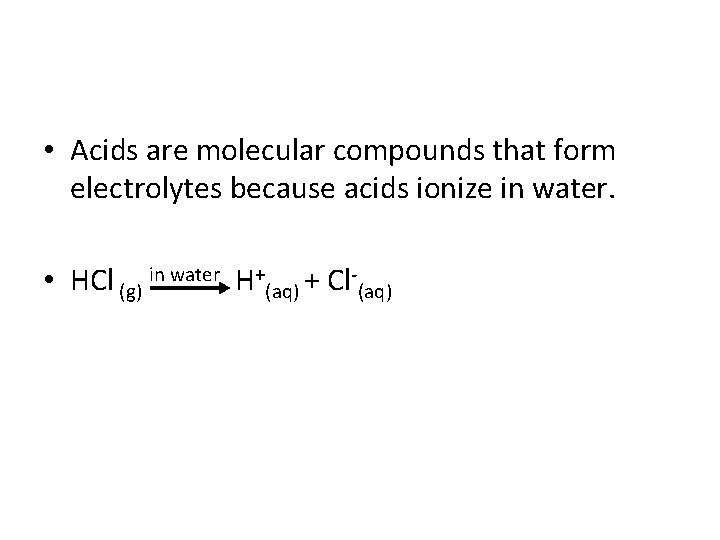
• Acids are molecular compounds that form electrolytes because acids ionize in water. • HCl (g) in water H+(aq) + Cl (aq)

Solutions and Chemical Reactions • In solution, solute particles separate, disperse, and collide with other solute particles. Reactions that would occur very slowly between undissolved solutes happen very quickly in solution.

• Do Section 5. 1 Review P. 175 #1, 3 -7

Section 5. 2 Solubility (P. 176 – 183) • Solubility - Solubility is the maximum amount of solute that will dissolve in a specific amount of solvent at a specific temperature. • For example: The solubility of sodium chloride in water at 20˚C is 36 g/100 m. L

• Saturated solutions – contain the maximum amount of dissolved solute at a given temperature in the presence of undissolved solute. A saturated solution may still be able to dissolve other solutes.

• Unsaturated solutions – can still dissolve more solute at a particular temperature

• Supersaturated solutions – contain more dissolved solute than its solubility at a given temperature. Supersaturated solutions are unstable.
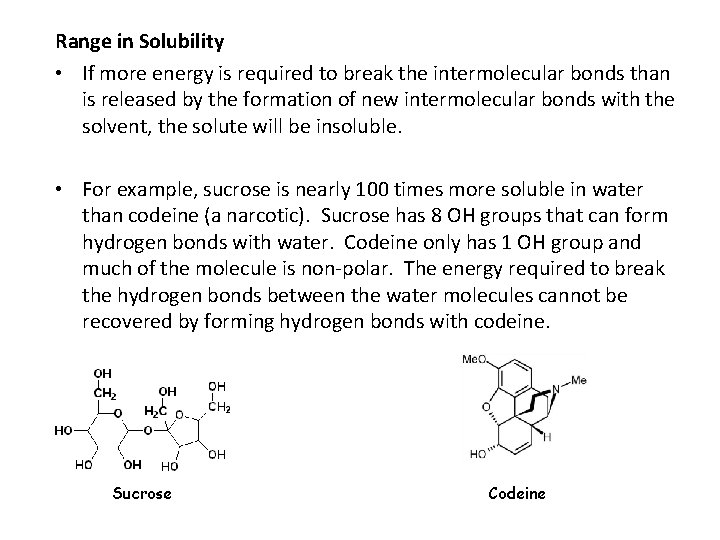
Range in Solubility • If more energy is required to break the intermolecular bonds than is released by the formation of new intermolecular bonds with the solvent, the solute will be insoluble. • For example, sucrose is nearly 100 times more soluble in water than codeine (a narcotic). Sucrose has 8 OH groups that can form hydrogen bonds with water. Codeine only has 1 OH group and much of the molecule is non polar. The energy required to break the hydrogen bonds between the water molecules cannot be recovered by forming hydrogen bonds with codeine. Sucrose Codeine
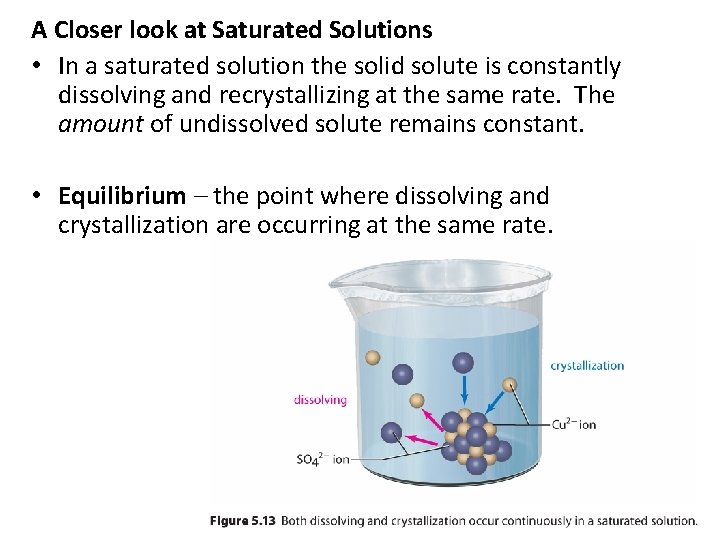
A Closer look at Saturated Solutions • In a saturated solution the solid solute is constantly dissolving and recrystallizing at the same rate. The amount of undissolved solute remains constant. • Equilibrium – the point where dissolving and crystallization are occurring at the same rate.
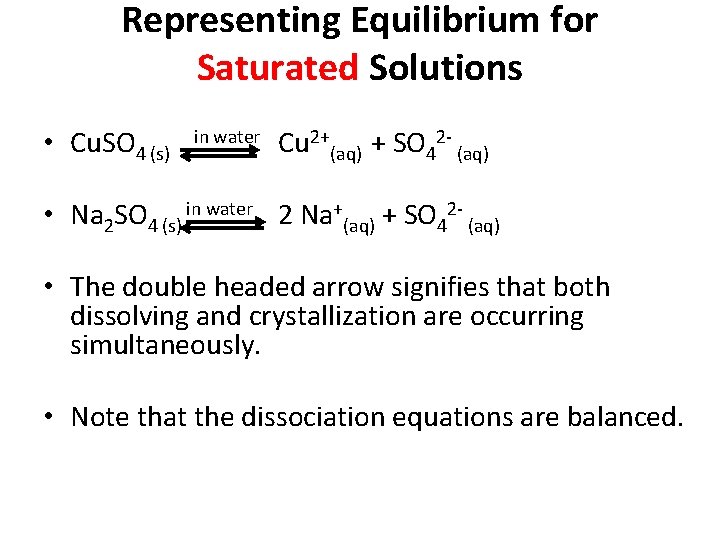
Representing Equilibrium for Saturated Solutions • Cu. SO 4 (s) in water Cu 2+(aq) + SO 42 (aq) • Na 2 SO 4 (s) in water 2 Na+(aq) + SO 42 (aq) • The double headed arrow signifies that both dissolving and crystallization are occurring simultaneously. • Note that the dissociation equations are balanced.

Solubility is affected by: • Temperature – most solids are more soluble at high temperatures because more energy is available to break the bonds holding the solid together. – The bonds holding liquids together are weaker than the bonds holding solids together and require less energy to break. Therefore, the solubility of most liquids is not affected by temperature. – Gas particles have a lot of Ek. When a gas dissolves in a liquid it loses some of its Ek. At higher temperatures the gas gains energy again and come out of solution. As a result gases are more soluble at low temperatures.

• Pressure – Changes in pressure have little effect of the solubility of solids and liquids. – Solubility of a gas in a liquid increases with an increase in pressure.

As a SCUBA diver swims deeper underwater the solubility of the nitrogen gas present in the air he breathes increases and more dissolves into his blood. As the diver returns to the surface the pressure decreases and some of the dissolved nitrogen gas comes out of solution. If the diver surfaces too quickly bubbles of nitrogen can form in the blood which could obstruct blood flow to the brain and muscles. Aka “the bends”

• Do 5. 2 Review P. 183 #1 -7

5. 3 The Concentration of Solutions (P. 184– 196) Concentration Expressions and Calculations • Concentration of a solution can be expressed quantitatively in a number of different ways:
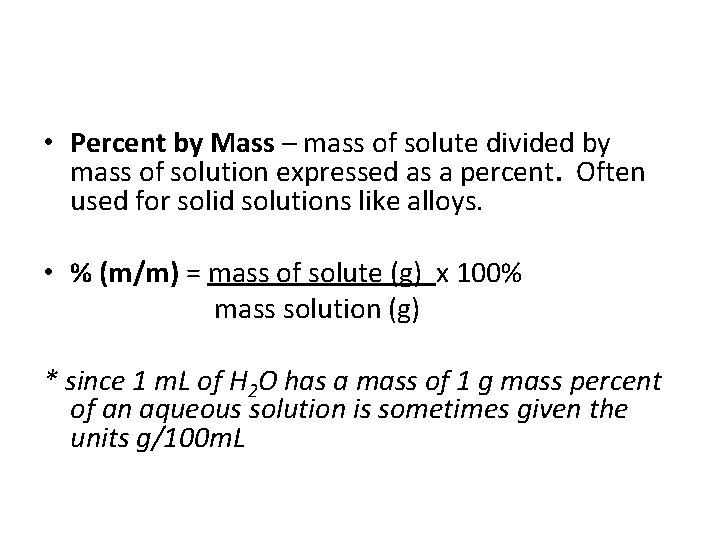
• Percent by Mass – mass of solute divided by mass of solution expressed as a percent. Often used for solid solutions like alloys. • % (m/m) = mass of solute (g) x 100% mass solution (g) * since 1 m. L of H 2 O has a mass of 1 g mass percent of an aqueous solution is sometimes given the units g/100 m. L

An easy example. . . • 0. 129 g of Mg dissolved to form 100 g of solution would be 0. 129% Mg • Or 0. 129 g/100 m. L Mg.

Example Calculations: • 1. Stainless steel contains 10. 5% chromium by mass. How much chromium is needed to produce 10 kg of stainless steel?
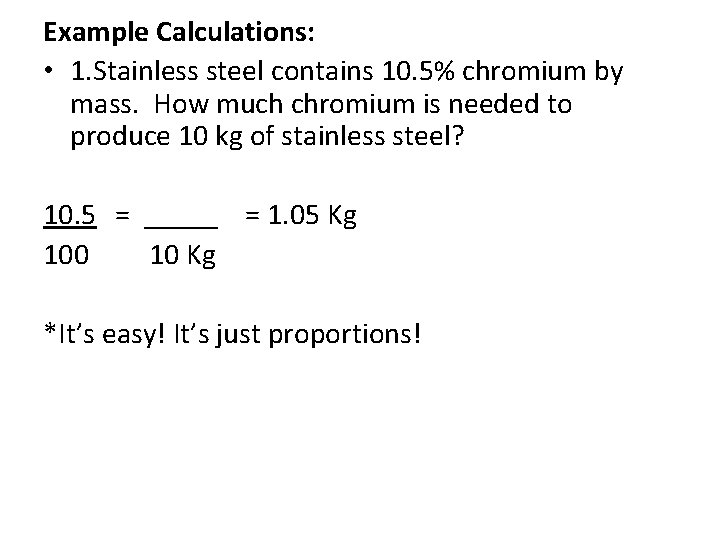
Example Calculations: • 1. Stainless steel contains 10. 5% chromium by mass. How much chromium is needed to produce 10 kg of stainless steel? 10. 5 = _____ = 1. 05 Kg 100 10 Kg *It’s easy! It’s just proportions!

• Remember, It is mass of solute over mass of solution, so … If 20. 0 g of solute are dissolved in 100 g of water, 20 g/120 g = 0. 167% Note that the total mass of the solution is 120 g (mass of solute + mass of solvent = mass of solution)

• 2. A 50. 0 m. L sample of water taken from the Dead Sea contains 15. 75 g of salt. What is the percent by mass concentration of salt in the Dead Sea?

• 2. A 50. 0 m. L sample of water taken from the Dead Sea contains 15. 75 g of salt. What is the percent by mass concentration of salt in the Dead Sea? 15. 75 g = ______ = 31. 5 g 50 m. L 100 m. L *Remember: % by mass = g/100 m. L in aqueous solutions.
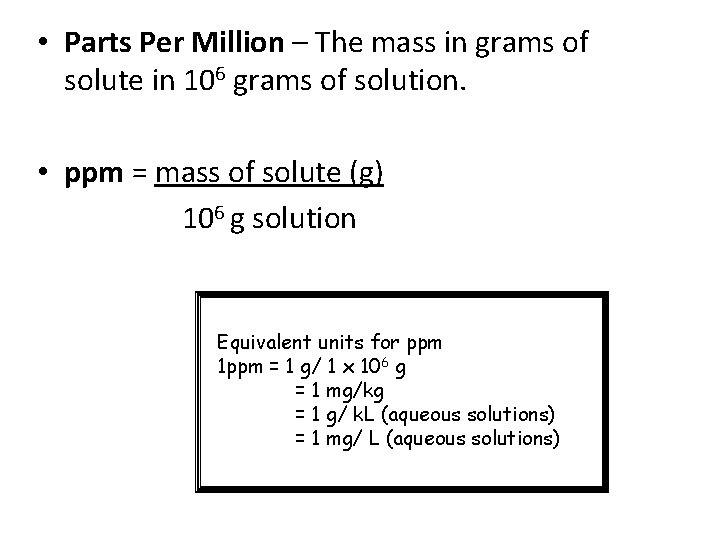
• Parts Per Million – The mass in grams of solute in 106 grams of solution. • ppm = mass of solute (g) 106 g solution Equivalent units for ppm 1 ppm = 1 g/ 1 x 106 g = 1 mg/kg = 1 g/ k. L (aqueous solutions) = 1 mg/ L (aqueous solutions)

• Parts Per Billion– The mass in grams of solute in 109 grams of solution. • ppb = mass of solute (g) 109 g of solution

Example Calculations: 1. What is the concentration of salt in the Dead Sea in ppm? • Since 1 m. L of water = 1 g of water. . .

15. 75 g = ______g 50 m. L 1 000 m. L Or you could convert your answer in % by mass to ppm. . . % by mass is like pph! 31. 5 = ____ 100 1 000 = 315 000 ppm = 3. 15 x 105 ppm

2. What is the concentration of salt in the Dead Sea in ppb?

2. What is the concentration of salt in the Dead Sea in ppb? Easy! Just move your decimal 3 spots! 315 000 = _315 000_ 1 000 000 = 3. 15 x 108 ppb
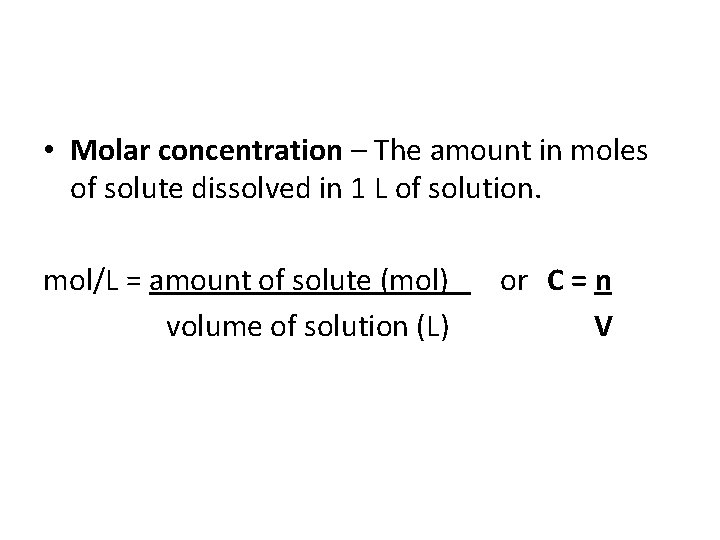
• Molar concentration – The amount in moles of solute dissolved in 1 L of solution. mol/L = amount of solute (mol) or C = n volume of solution (L) V

• Molar concentration is used to solve problems involving chemical reactions. Since molar concentration is used so frequently by chemists a special short hand is used. For example: [Na. Cl(aq)] = 0. 10 mol/L

Example calculations: • What is the concentration of a solution containing 0. 350 mol of H 2 SO 4(aq) in 750 m. L of water? C = n V
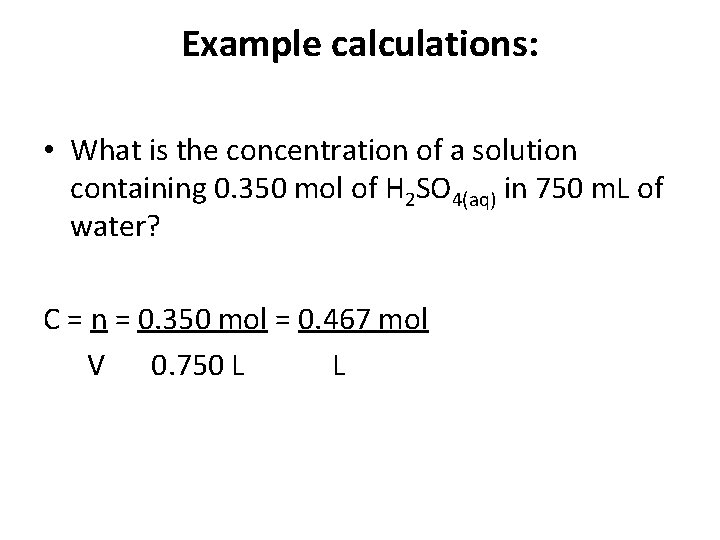
Example calculations: • What is the concentration of a solution containing 0. 350 mol of H 2 SO 4(aq) in 750 m. L of water? C = n = 0. 350 mol = 0. 467 mol V 0. 750 L L

• What is the concentration when 200 g of sodium sulfate is dissolved in 3. 00 L of water?

• What is the concentration when 200 g of sodium sulfate is dissolved in 3. 00 L of water? Na 2 SO 4 M= (2 x 22. 99 g/mol) + (1 x 32. 06 g/mol) + (4 x 16. 00 g/mol) = 142. 04 g/mol n = m = 200 g = 1. 41 mol M 142. 04 g/mol
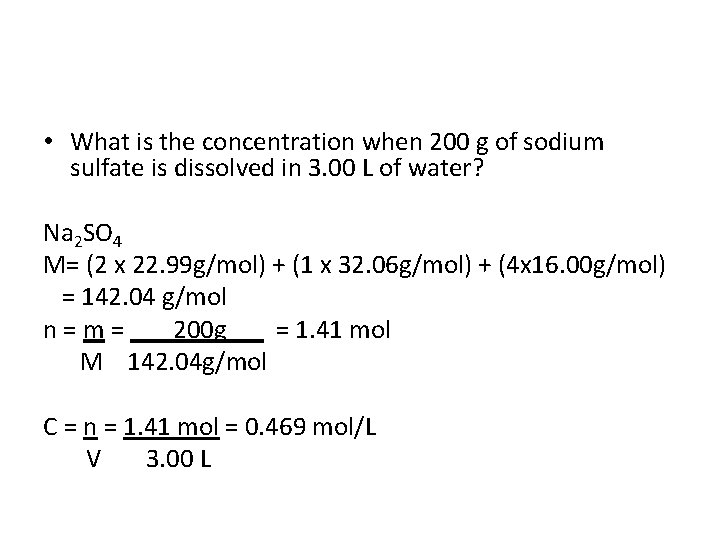
• What is the concentration when 200 g of sodium sulfate is dissolved in 3. 00 L of water? Na 2 SO 4 M= (2 x 22. 99 g/mol) + (1 x 32. 06 g/mol) + (4 x 16. 00 g/mol) = 142. 04 g/mol n = m = 200 g = 1. 41 mol M 142. 04 g/mol C = n = 1. 41 mol = 0. 469 mol/L V 3. 00 L

• What volume of a 0. 200 mol/L solution of phosphoric acid contains 0. 175 moles of acid?

• What volume of a 0. 200 mol/L solution of phosphoric acid contains 0. 175 moles of acid? V = n = 0. 175 mol = 0. 875 L C 0. 200 mol/L

• How many moles of salt would be found in 1. 5 GL of a 0. 100 mol/L solution of sodium chloride? 1 GL = 1 000 000 L or 109 L

• How many moles of salt would be found in 1. 5 GL of a 0. 100 mol/L solution of sodium chloride? 1 GL = 1 000 000 L or 109 L n = V x C = (1. 5 x 109 L)(0. 100 mol/L) = 1. 5 x 108 mol

• What mass of potassium chloride is required to prepare 700 m. L of a 2. 50 mol/L solution?
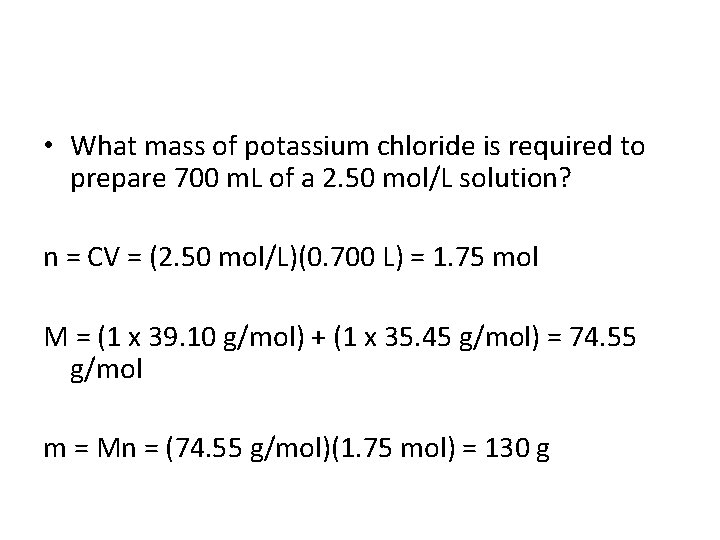
• What mass of potassium chloride is required to prepare 700 m. L of a 2. 50 mol/L solution? n = CV = (2. 50 mol/L)(0. 700 L) = 1. 75 mol M = (1 x 39. 10 g/mol) + (1 x 35. 45 g/mol) = 74. 55 g/mol m = Mn = (74. 55 g/mol)(1. 75 mol) = 130 g

• Do Practice Problems P. 186 #1 -5, P. 188 #6 -10 and P. 191 #11 -16

Molar Concentration of Ions in Solution • Ionic compounds in solution dissociate into individual ions. You will sometimes be asked to calculate the concentration of individual ions in solution, not just the solute.
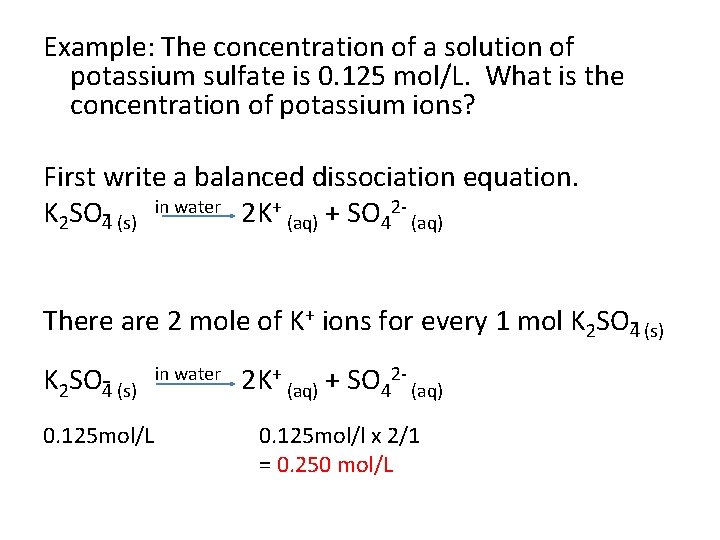
Example: The concentration of a solution of potassium sulfate is 0. 125 mol/L. What is the concentration of potassium ions? First write a balanced dissociation equation. K 2 SO 4 (s) in water 2 K+ (aq) + SO 42 (aq) There are 2 mole of K+ ions for every 1 mol K 2 SO 4 (s) in water 2 K+ (aq) + SO 42 (aq) 0. 125 mol/L 0. 125 mol/l x 2/1 = 0. 250 mol/L
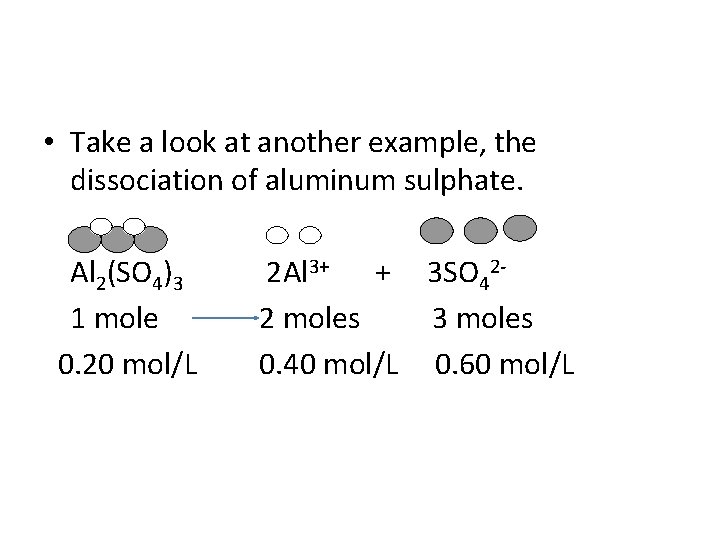
• Take a look at another example, the dissociation of aluminum sulphate. Al 2(SO 4)3 2 Al 3+ + 3 SO 42 1 mole 2 moles 3 moles 0. 20 mol/L 0. 40 mol/L 0. 60 mol/L
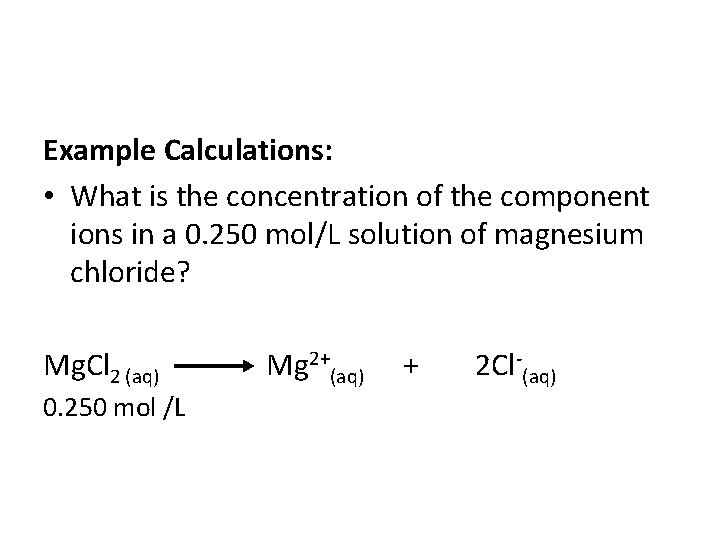
Example Calculations: • What is the concentration of the component ions in a 0. 250 mol/L solution of magnesium chloride? Mg. Cl 2 (aq) Mg 2+(aq) + 2 Cl (aq) 0. 250 mol /L
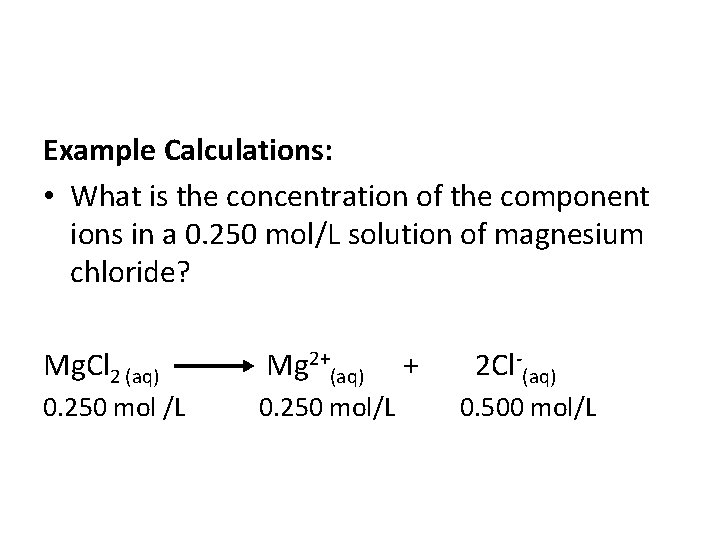
Example Calculations: • What is the concentration of the component ions in a 0. 250 mol/L solution of magnesium chloride? Mg. Cl 2 (aq) Mg 2+(aq) + 2 Cl (aq) 0. 250 mol /L 0. 250 mol/L 0. 500 mol/L
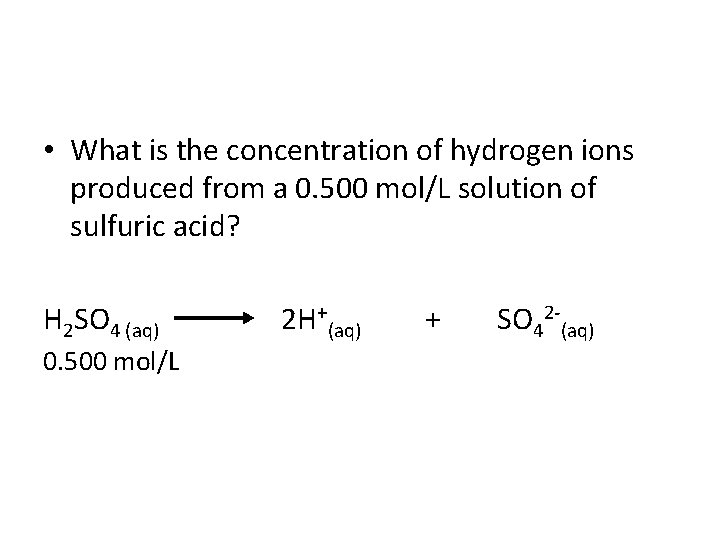
• What is the concentration of hydrogen ions produced from a 0. 500 mol/L solution of sulfuric acid? H 2 SO 4 (aq) 2 H+(aq) + SO 42 (aq) 0. 500 mol/L
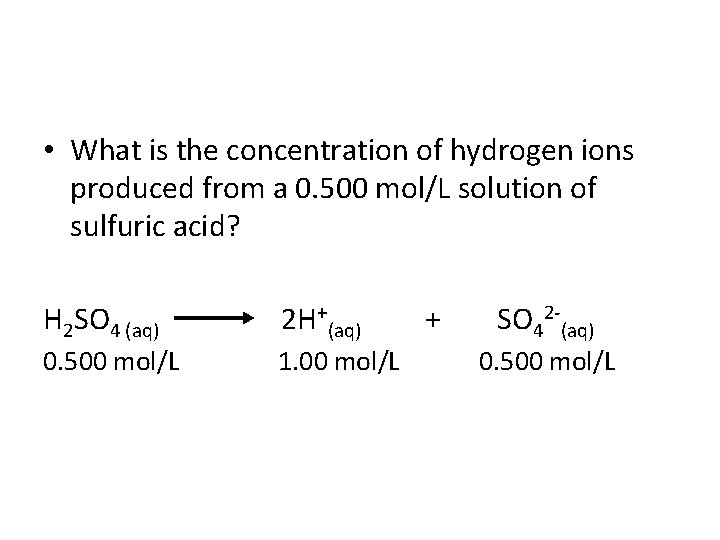
• What is the concentration of hydrogen ions produced from a 0. 500 mol/L solution of sulfuric acid? H 2 SO 4 (aq) 2 H+(aq) + SO 42 (aq) 0. 500 mol/L 1. 00 mol/L 0. 500 mol/L

• Do Practice Problems P. 193 #17 -22, P. 194 #23 -26, and P. 195 #27 -30 • Do Section 5. 3 Review P. 196 #1 -11, 14, and 15

Section 5. 4 Preparing and Diluting Solutions (P. 197– 202) • A solution with a known concentration is called a standard solution. • A standard solution can be made by either dissolving a measured mass of solute in a certain volume of solution or diluting another standard solution by adding a known volume of additional solvent.
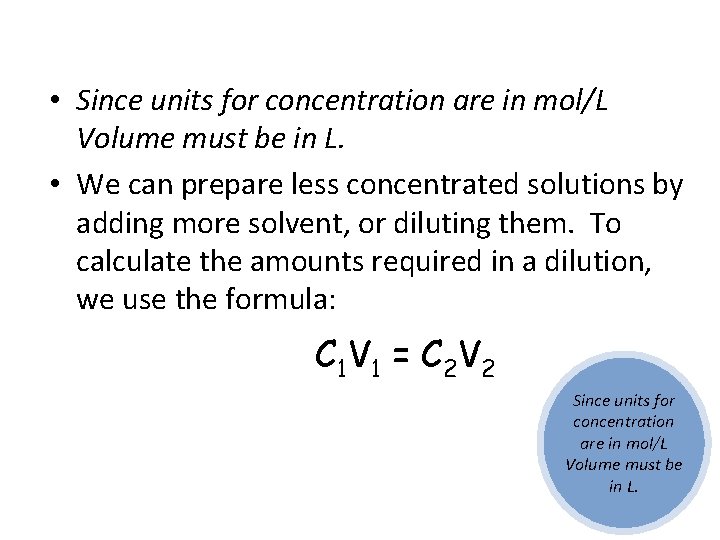
• Since units for concentration are in mol/L Volume must be in L. • We can prepare less concentrated solutions by adding more solvent, or diluting them. To calculate the amounts required in a dilution, we use the formula: C 1 V 1 = C 2 V 2 Since units for concentration are in mol/L Volume must be in L.

Example Calculations: • What is the final concentration of 10. 0 m. L of a 2. 00 mol/L solution if it is diluted to 800 m. L?
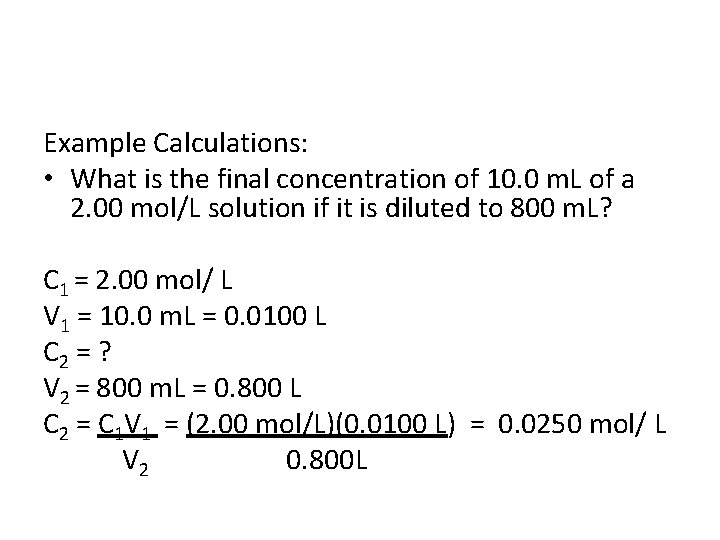
Example Calculations: • What is the final concentration of 10. 0 m. L of a 2. 00 mol/L solution if it is diluted to 800 m. L? C 1 = 2. 00 mol/ L V 1 = 10. 0 m. L = 0. 0100 L C 2 = ? V 2 = 800 m. L = 0. 800 L C 2 = C 1 V 1 = (2. 00 mol/L)(0. 0100 L) = 0. 0250 mol/ L V 2 0. 800 L
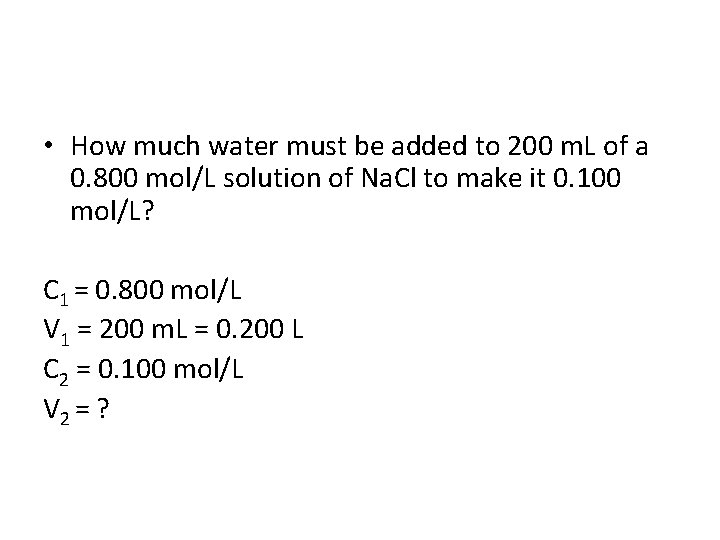
• How much water must be added to 200 m. L of a 0. 800 mol/L solution of Na. Cl to make it 0. 100 mol/L? C 1 = 0. 800 mol/L V 1 = 200 m. L = 0. 200 L C 2 = 0. 100 mol/L V 2 = ?

• How much water must be added to 200 m. L of a 0. 800 mol/L solution of Na. Cl to make it 0. 100 mol/L? C 1 = 0. 800 mol/L V 1 = 200 m. L = 0. 200 L C 2 = 0. 100 mol/L V 2 = ? V 2 = C 1 V 1 = (0. 800 mol/L)(0. 200 L) = 1. 60 L C 2 0. 100 mol/L 1. 60 L – 0. 200 L = 1. 40 L or 1400 m. L must be added.

• What concentration was the initial solution of Na. HCO 3 if 100 m. L is diluted to produce 1000 m. L of a 0. 0400 mol/L solution? C 1 = ? V 1 = 100 m. L = 0. 100 L C 2 = 0. 0400 mol/L V 2 = 1000 m. L = 1 L
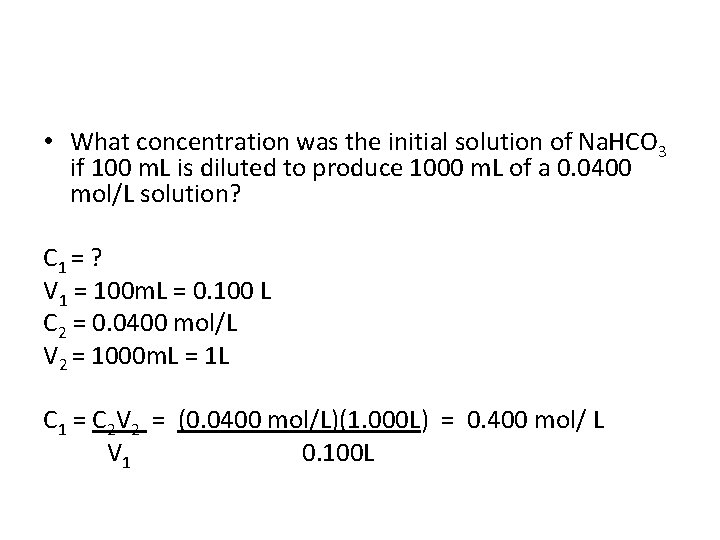
• What concentration was the initial solution of Na. HCO 3 if 100 m. L is diluted to produce 1000 m. L of a 0. 0400 mol/L solution? C 1 = ? V 1 = 100 m. L = 0. 100 L C 2 = 0. 0400 mol/L V 2 = 1000 m. L = 1 L C 1 = C 2 V 2 = (0. 0400 mol/L)(1. 000 L) = 0. 400 mol/ L V 1 0. 100 L

• Do Practice Problems P. 198 #31 -33 • Do Section 5. 4 Review # 2 -4, 7 -11 • Do Chapter 5 Review P. 204 # 2 -6, 8, 9, 13 -18, 20, 22, 25, and 30
- Slides: 79