UNIT 3 ELECTRONS AND THE PERIODIC TABLE Chapter

UNIT 3 ELECTRONS AND THE PERIODIC TABLE Chapter 5 & 6

CHAPTER 5: ELECTRONS IN ATOMS

WHAT ARE THE CHEMICAL PROPERTIES OF ATOMS AND MOLECULES RELATED TO? • ELECTRONS! • Number of valence electrons • How much an atom wants to “gain” or “lose” electrons • How many electrons “gained” or “lost”
ELECTRON HISTORY • Dalton’s Model • Didn’t even know of electrons or charge – just ratios of atoms. • Thomson’s Model • ELECTRONS EXIST!!!! • Where are they? No clue. • Plum Pudding? • Rutherford’s Model • Gold Foil Experiment • Electrons are found outside the nucleus. • Now knows where they are, but still doesn’t know how they are organized.
Rutherford’s model
BOHR’S MODEL • Electron’s are found in energy levels. Quantum: Specific energy values that have exact values in between them. • Pictured the atom like a solar system with electrons “orbiting” around the nucleus. • Each orbit path associated with a particular energy. • Now a better understanding of how electrons are organized, but it still isn’t quite correct.
QUANTUM MECHANICAL MODEL ERWIN SCHRODINER (1926) • Electrons are found in regions of empty space around the nucleus. “electron clouds”. • Shape of the region corresponds to different energy levels. • Shape of the region represents the probability of the electron’s location 90% of the time. • PROBLEM? These are still called “atomic orbitals”.
SCHRODINGER’S MODEL • Electrons are “wave functions”. • We can’t actually know exactly where an electron is at any moment. • We can know a probability of an electrons location based on its energy. • Ex: a windmill blade
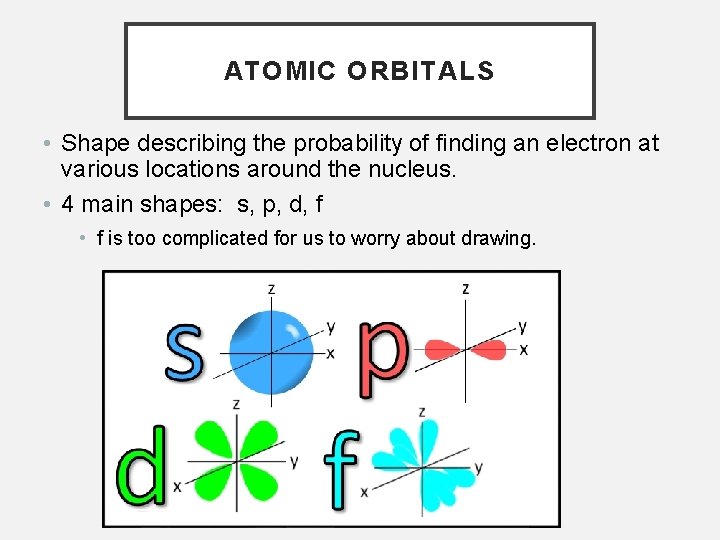
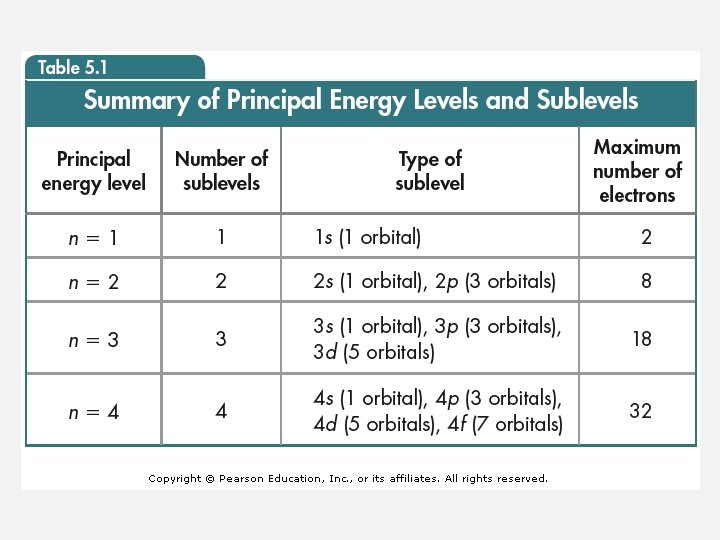
ATOMIC ORBITALS • Shape describing the probability of finding an electron at various locations around the nucleus. • 4 main shapes: s, p, d, f • f is too complicated for us to worry about drawing.
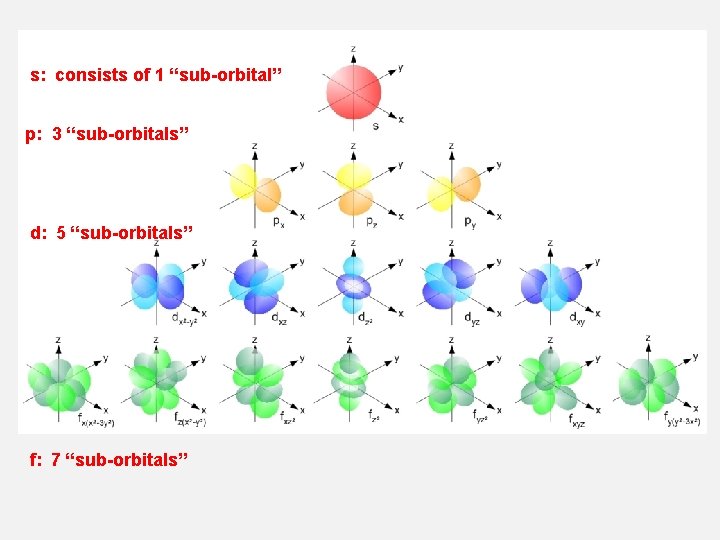
s: consists of 1 “sub-orbital” p: 3 “sub-orbitals” d: 5 “sub-orbitals” f: 7 “sub-orbitals”
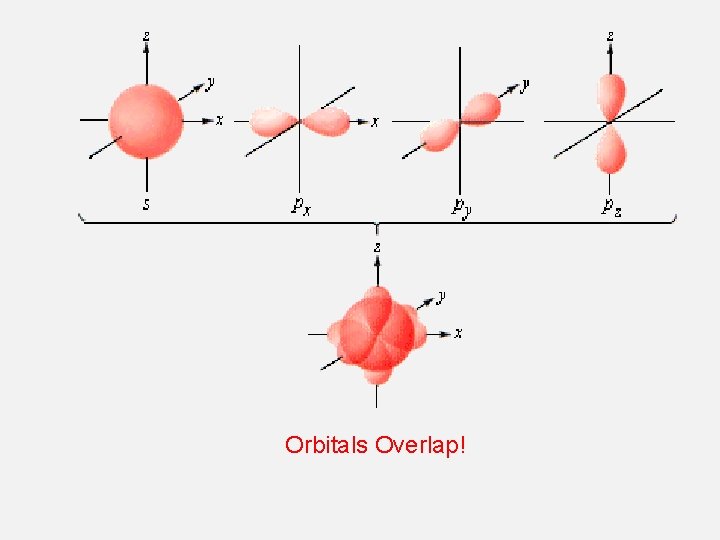
Orbitals Overlap!
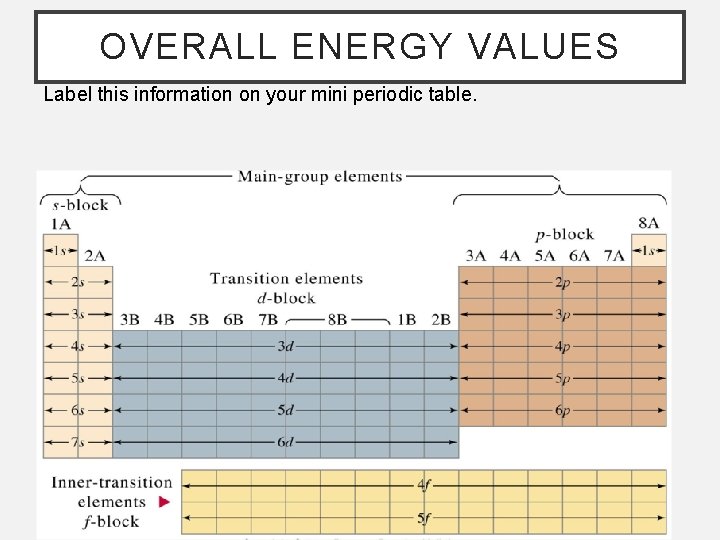
OVERALL ENERGY VALUES Label this information on your mini periodic table.
ELECTRON CONFIGURATIONS • How electrons are arranged in various orbitals around the nucleus. • Three rules explain how: Aufbau Principle, Pauli Exclusion Principle, and Hund’s rule.
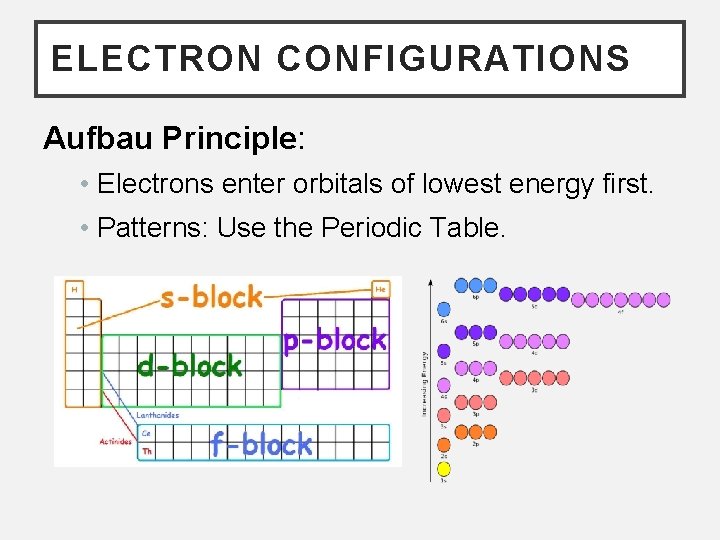
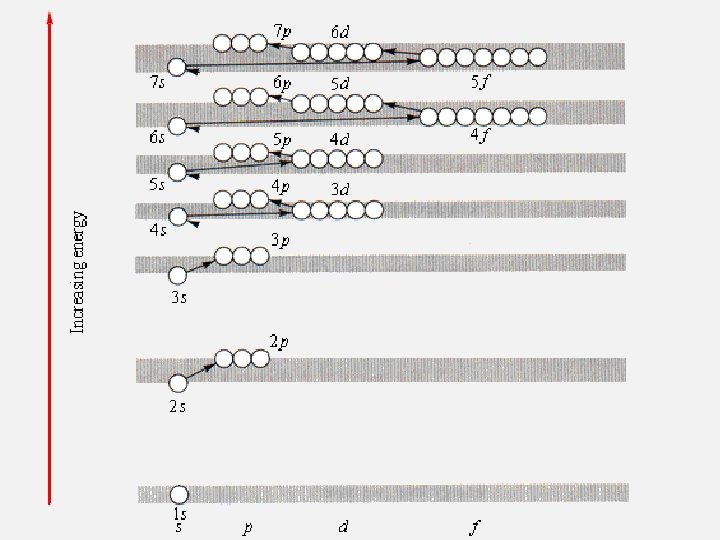
ELECTRON CONFIGURATIONS Aufbau Principle: • Electrons enter orbitals of lowest energy first. • Patterns: Use the Periodic Table.
ELECTRON CONFIGURATIONS Pauli Exclusion Principle: • Only 2 electrons per orbital • Each orbital contains electrons of opposite spin. (up arrow, or down arrow)
ELECTRON CONFIGURATIONS Hunds Rule: • When electrons enter orbitals of equal energy, all the sublevels are filled with 1 electron of the same spin first. • Example: • P’s (3 total sublevels) • Each of the 3 sublevels will fill with only 1 electron first • Then they will begin to fill with the second electron of opposite spin.
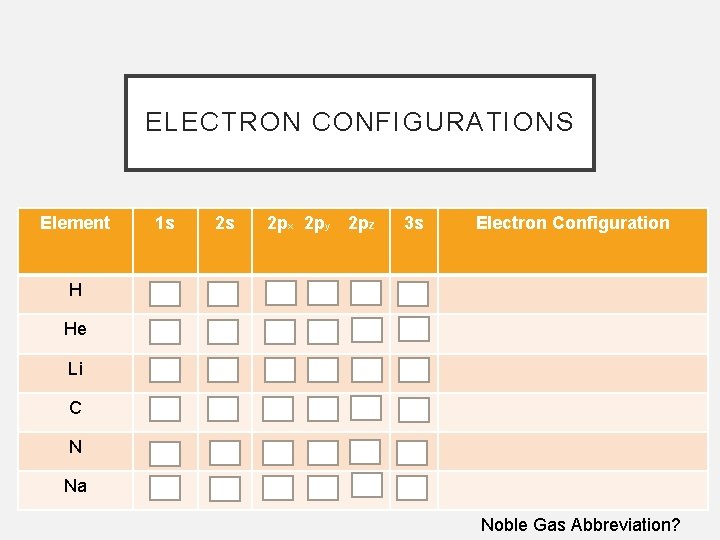
ELECTRON CONFIGURATIONS Element 1 s 2 s 2 px 2 py 2 pz 3 s Electron Configuration H He Li C N Na Noble Gas Abbreviation?
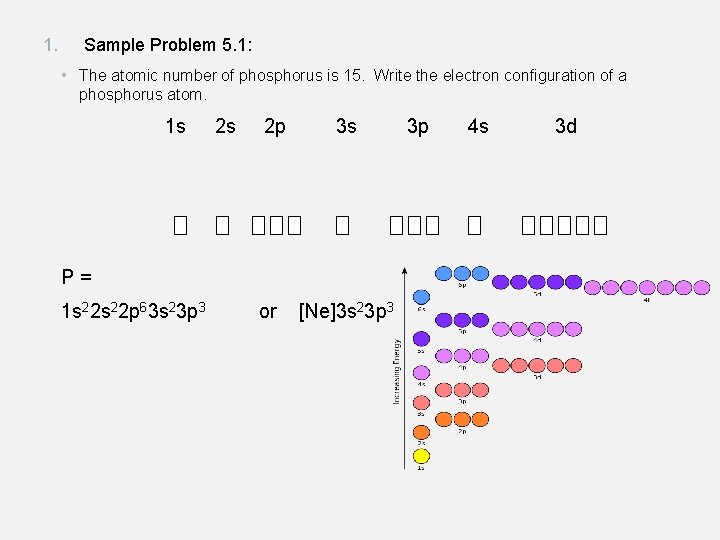
1. Sample Problem 5. 1: • The atomic number of phosphorus is 15. Write the electron configuration of a phosphorus atom. 1 s 2 s 2 p 3 s P= 1 s 22 p 63 s 23 p 3 or [Ne]3 s 23 p 3 3 p 4 s 3 d
2. Complete the following Electron Configurations Carbon Sulfur Full Electron Configuration Abbreviated Configuration
2. Complete the following Electron Configurations Nickel Krypton Full Electron Configuration Abbreviated Configuration

3. Why do we care about electron configurations? • Chemical properties are related to electrons. • They explain the organization of electrons. 4. What is the expected electron configuration of Mo? • 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 4 Fill this into a detailed orbital diagram. 5. The actual electron configuration for Mo is: • 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 14 d 5 Fill this into a detailed orbital diagram 6. Why is Mo’s electron configuration different than expected? • An (s) orbital electron gets “promoted” to the d orbitals • Hund’s Rule: All 5 d orbitals with 1 electron is more “stable” than having 1 of the d orbitals empty.

1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 14 d 5 7. How many unpaired e-s are in the electron configuration above? • (6) 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 14 d 5 8. Which e-s are most energetic? Least energetic? • 5 s 1 are most energetic (4 d 5 are close). • They are lower in energy and NOT VALENCE. They are more strongly attracted to the nucleus. • All the others are LOWER in Energy. 9. How could you abbreviate the electron configuration above? • [Kr] 5 s 14 d 5 10. Why would you abbreviate and leave off the “inner-core” electrons? 11. What other elements would you predict have electron configurations like Mo? • Cr & W (Same COLUMN)

12. Which electrons are usually closest to the nucleus in the following: 1 s 22 p 63 s 23 p 64 s 1 • 1 s 22 p 63 s 23 p 6 (the “inner core” energy level electrons) 13. Which electrons are furthest from the nucleus? • 4 s 1 (the highest principle” energy level electrons) 14. Which electrons would you think would be easiest to remove from the atom above? Why? • 4 s 1 (The electrons farther from the nucleus are attracted with a smaller force to the nucleus. ) 15. What name is given to these “outermost” electrons? • VALENCE electrons

IONS AND ELECTRONS • Ions have additional or missing electrons. • These electrons fill or come from the outermost energy level. • Electrons gained or lost are VALENCE ELECTRONS. Anions: Non-metals that gain electrons. Cations: Metals that lose electrons. • Example: O 2 - Anion • has 10 electrons; gained 2 electrons • 1 s 22 p 6 • same electron configuration as [Ne]
16. Give the Full & noble gas (abbrev) GROUND STATE electron configuration for the following ions: • O 2 - • Cu 2+ • N 3 - • Cu 1+ (Special Case)***

UNIT 3: ELECTRONS AND THE PERIODIC TABLE Agenda: Friday 11/3 • Warm-up • Electrons and Light Notes • Work on Worksheet 3

WARM-UP 11/3 1. Which element has the following electron configurations? A. 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 1 B. [Kr] 5 s 24 d 9 2. DEMO Emission Spectra of Elements: Why do elements give off different colors of light when their electrons get excited? You. Tube

17. What keeps an electron bound to an atom? 18. In the space below, build an atom that contains 3 protons, 4 neutrons and 3 electrons. 19. How many valence electrons are in the atom above? 20. Predict what would happen when the valance (outermost) electron absorbed some energy from an outside source. 21. What would happen when that electron loses that energy that it absorbed? 22. How are different colors of light formed?
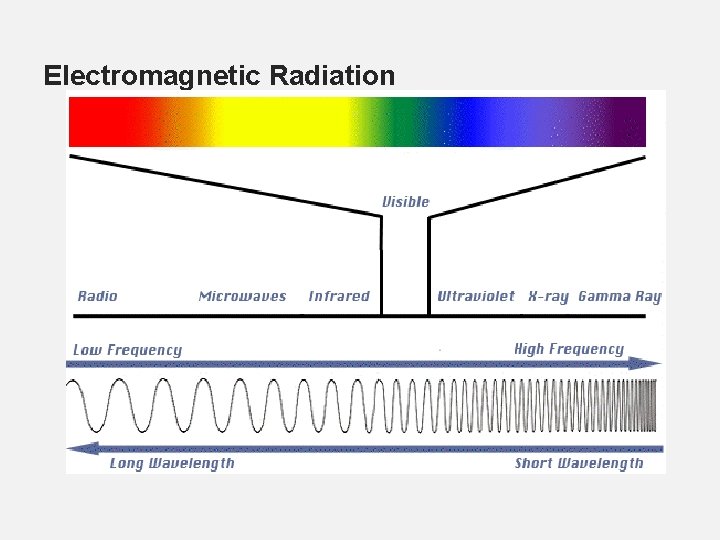
Electromagnetic Radiation
23. Describe how light is created. 24. List the different forms of visible light in order of lowest energy to greatest energy. 25. What color of visible light would be the most appropriate to use if you are star gazing at night and do not want to disturb other star gazers? Why? 26. Predict the color of visible light produced in the following situations: • A 3 s electron jumps to the 4 th energy level as it absorbs light, then falls back down to the ground state. • A 3 s electron jumps to the 7 th energy level as it absorbs light, then falls back down to the ground state. • A 5 p electron absorbs energy and jumps to the 6 th energy level. • Youtube: Flame test lab

UNIT 3: ELECTRONS AND THE PERIODIC TABLE Agenda: Monday 11/6 • Flame Test Activity

UNIT 3: ELECTRONS AND THE PERIODIC TABLE Agenda: Tuesday 11/7 • HW 1 Quiz • Chapter 6 Notes (Periodic Table) • Work on Worksheet 4 or Homework 2

6. 1 ORGANIZING THE ELEMENTS PERIODIC TABLE HISTORY • Dmitri Mendeleev – mid 1800’s • Proposed a table for 70 elements based on mass and properties • Henry Moseley – 1913 • Determined the atomic number of elements and arranged the table in order of atomic number
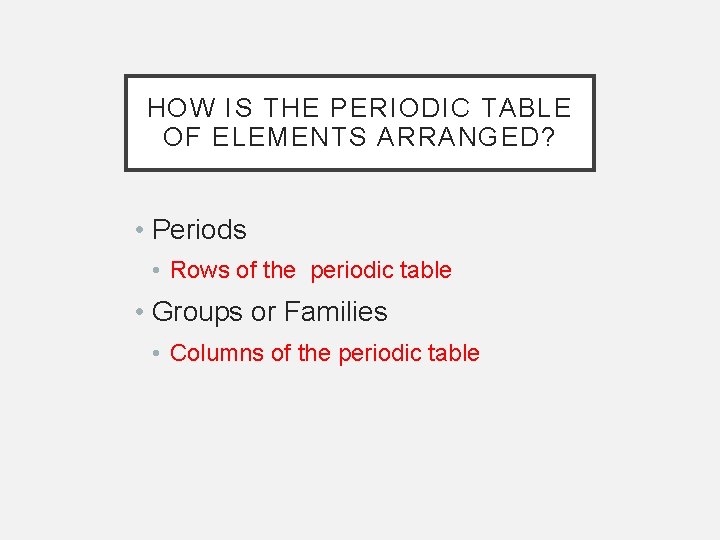
HOW IS THE PERIODIC TABLE OF ELEMENTS ARRANGED? • Periods • Rows of the periodic table • Groups or Families • Columns of the periodic table

• Periodic Law: • The elements are arranged in order of increasing atomic number • There is a periodic repetition of their physical and chemical properties • Elements with similar properties end up in the same column • Basis of Law: • The placement of elements into families (columns) results from them having the same outermost electron patterns.
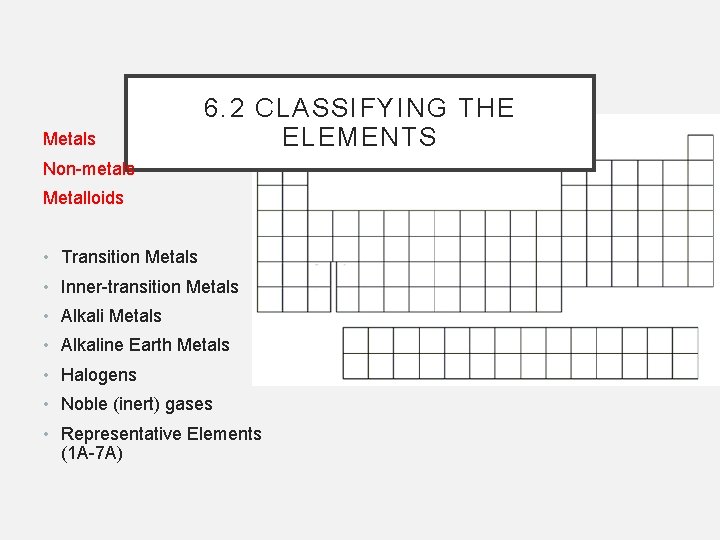
Metals 6. 2 CLASSIFYING THE ELEMENTS Non-metals Metalloids • Transition Metals • Inner-transition Metals • Alkaline Earth Metals • Halogens • Noble (inert) gases • Representative Elements (1 A-7 A)
CHEMICAL PROPERTIES & FAMILIES • Chemical Properties of elements are based on their “Valence Electrons” • Families are groups of Elements that have similar “Valence Electrons”

• Valence Electrons • Outermost electrons in an atom. • Types of Ions • Cations: Lose electrons to become POSITIVE • Anions: Gain electrons to become NEGATIVE
Valence Electron Patterns Ion Type Patterns

UNIT 3: ELECTRONS AND THE PERIODIC TABLE Agenda: Block 2 (11/8 or 11/9) • HW 2 Quiz • Finish Chapter 6 Notes (Periodic Trends) • Finish Worksheet 4 • Work on HW 3
6. 3 PERIODIC TRENDS: ATOMIC RADIUS • In general, the more VALENCE electrons, the smaller the size of the atoms electron clouds. • Exception: Hydrogen and Helium • However, adding periods increases the size of the atom. • WHY? Nuclear Charge and Principle Energy Level
PERIODIC TRENDS: ION RADIUS • - Ions (Anions) have full VALENCE electrons = LARGER than their ATOM SKIP • + Ions (Cations) have empty VALENCE electrons = SMALLER than their ATOM • BUT…. The size of one ion compared to the next is the same pattern as ATOMIC RADIUS
PERIODIC TRENDS: 1 ST IONIZATION ENERGY • The amount of energy required to remove an electron from an atom. • Atoms that tend to LOSE electrons have LOW Ionization energy. • Lowest = Francium • Atoms that do not lose electrons easily have HIGH Ionization energy. • Highest = Helium • WHY? How much is the electron attracted to Nucleus?
PERIODIC TRENDS: ELECTRONEGATIVITY • The tendency for an atom to attract electrons of another atom. • (-) Anions that gain electrons have high electronegativity values (they desire more electrons) • Noble Gasses have very small electronegativities. • Examples • Highest = Fluorine • Lowest = Francium (NONNoble gas) • Lowest group = Noble gasses

UNIT 3: ELECTRONS AND THE PERIODIC TABLE Agenda: Monday 11/13 • Stamp WS 4 • HW 3 Quiz • Review • Unit Test Next Class

WARM-UP THURSDAY 10/26 1. At your table, discuss atomic structure. What part of an atom is most likely to interact with other atoms? 2. What is an ION? 3. What is a VALENCE electron?
- Slides: 48