Unit 3 Electrochemistry Electrochemistry When there is a












- Slides: 18

Unit 3: Electrochemistry

Electrochemistry � When there is a chemical reaction, electrons are involved. � During a chemical reaction, the substances that come in contact compete for each other’s electrons.

� The element that is the most active will take the electrons from the element that is the least reactive. � Electrons flow from one place to another to produce electricity. � Electrochemistry is the process of using electricity to force chemical reactions.

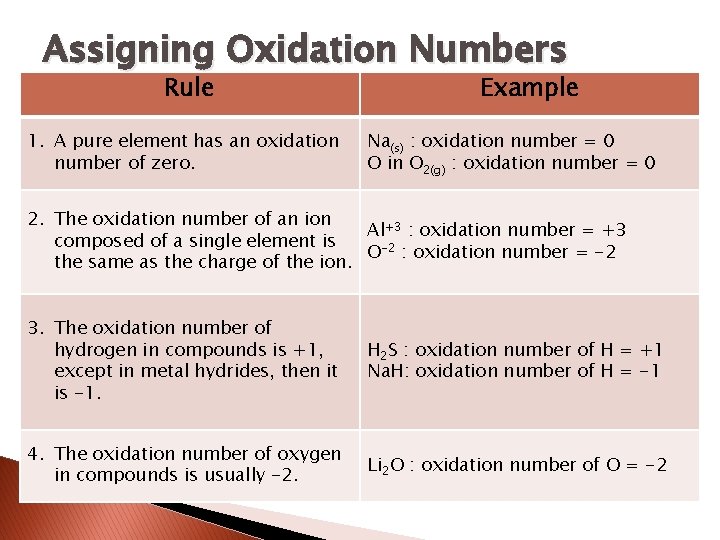
Oxidation Numbers � To identify redox reactions, it helps to be able to track what is happening to the electrons. � An oxidation number is a number assigned to an individual atom or ion in a substance using the following rules.

Assigning Oxidation Numbers Rule 1. A pure element has an oxidation number of zero. Example Na(s) : oxidation number = 0 O in O 2(g) : oxidation number = 0 2. The oxidation number of an ion Al+3 : oxidation number = +3 composed of a single element is O-2 : oxidation number = -2 the same as the charge of the ion. 3. The oxidation number of hydrogen in compounds is +1, except in metal hydrides, then it is -1. H 2 S : oxidation number of H = +1 Na. H: oxidation number of H = -1 4. The oxidation number of oxygen in compounds is usually -2. Li 2 O : oxidation number of O = -2

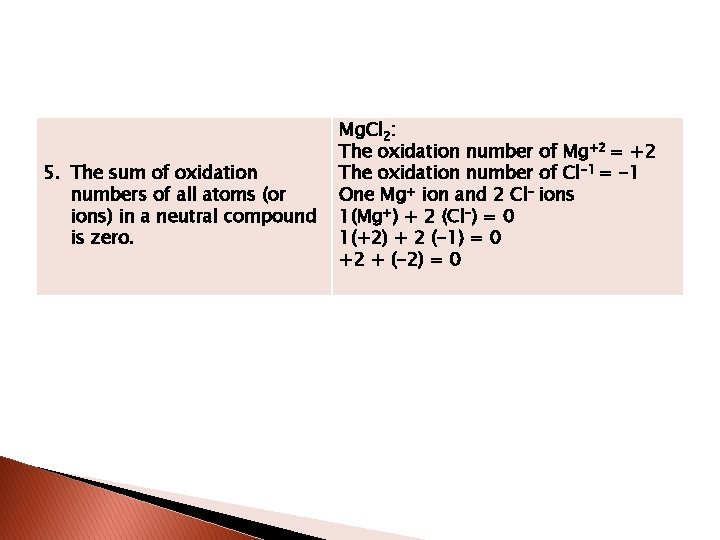
5. The sum of oxidation numbers of all atoms (or ions) in a neutral compound is zero. Mg. Cl 2: The oxidation number of Mg+2 = +2 The oxidation number of Cl-1 = -1 One Mg+ ion and 2 Cl- ions 1(Mg+) + 2 (Cl-) = 0 1(+2) + 2 (-1) = 0 +2 + (-2) = 0

Examples: What is the oxidation number for each of the following? � Zn(s) ◦ oxidation number = zero � N 2(g) ◦ oxidation number = zero � Cr+3(aq) ◦ oxidation number = +3

� KCl(s) ◦ oxidation number of K+1 = +1 ◦ oxidation number of Cl-1 = -1 � Al 2 O 3(s) ◦ oxidation number of Al+3 = +3 ◦ oxidation number of O-2 = -2 2(Al+3) + 3 (O-2) = 0 2(+3) + 3 (-2) = 0 +6 + (-6) = 0

Oxidation-Reduction Reactions � Oxidation is when a chemical loses electrons. The chemical has been oxidized. � Reduction is when a chemical gains electrons. The chemical has been reduced.


� Reactions when one reactant is oxidized and the other reactant is reduced are called an oxidation-reduction reaction, or a Redox reaction for short. � In a Redox reaction the number of electrons gained and lost must be equal.

Example: Identify the reactant oxidized and the reactant reduced in the following reaction: �Zn(s)+ Cu. SO 4(aq) Cu(s) + Zn. SO 4(aq)

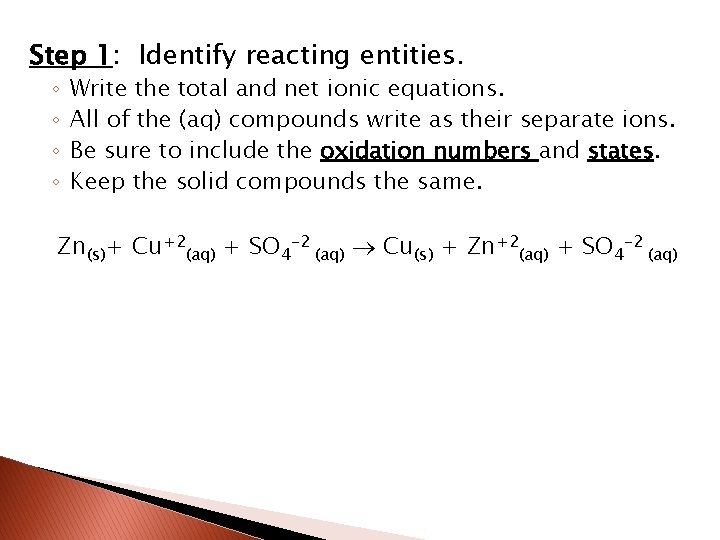
Step 1: Identify reacting entities. ◦ ◦ Write the total and net ionic equations. All of the (aq) compounds write as their separate ions. Be sure to include the oxidation numbers and states. Keep the solid compounds the same. Zn(s)+ Cu+2(aq) + SO 4 -2 (aq) Cu(s) + Zn+2(aq) + SO 4 -2 (aq)

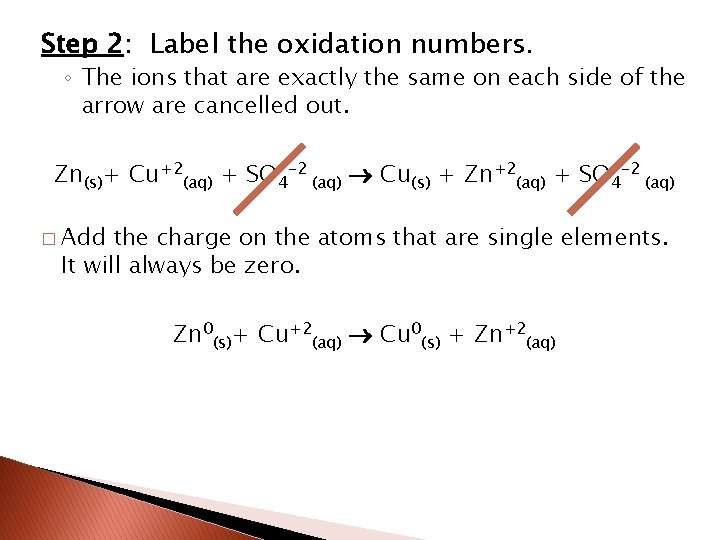
Step 2: Label the oxidation numbers. ◦ The ions that are exactly the same on each side of the arrow are cancelled out. Zn(s)+ Cu+2(aq) + SO 4 -2 (aq) Cu(s) + Zn+2(aq) + SO 4 -2 (aq) � Add the charge on the atoms that are single elements. It will always be zero. Zn 0(s)+ Cu+2(aq) Cu 0(s) + Zn+2(aq)

� Step 3: Identify loss and gain of electrons. Zn 0(s) becomes Zn+2(aq) ◦ a loss of 2 e◦ zinc is oxidized Cu+2(aq) becomes Cu 0(s) ◦ a gain of 2 e◦ copper is reduced

Summary of Redox Reactions � The charge on each atom in any lone element is always zero. � In a Redox reaction, electrons are transferred from one reactant to another reactant.

� Oxidation refers to the loss of electrons. Reactants that lose electrons are oxidized. � Reduction refers to the gain of electrons. Reactants that gain electrons are reduced. � Redox reactions occur in nature. Fruit turning brown when exposed to air is an example of a natural Redox reaction.

Handout “Identifying Oxidation and Reduction”