Unit 3 Chemical Reactions Review Questions You will



















- Slides: 28

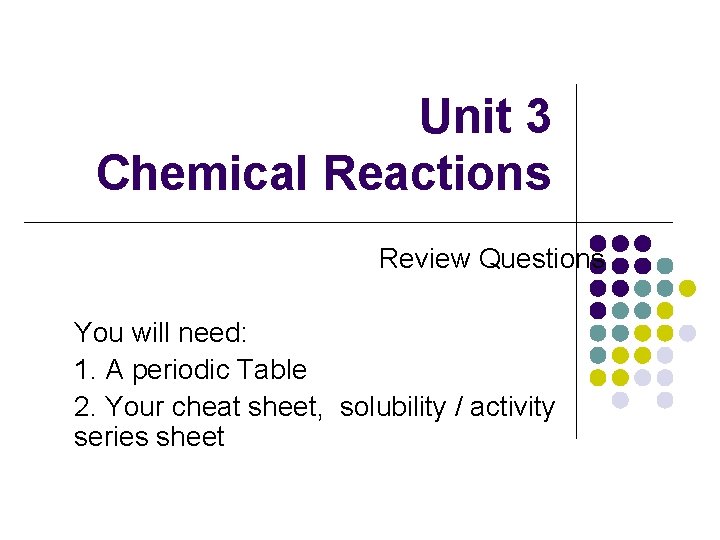
Unit 3 Chemical Reactions Review Questions You will need: 1. A periodic Table 2. Your cheat sheet, solubility / activity series sheet

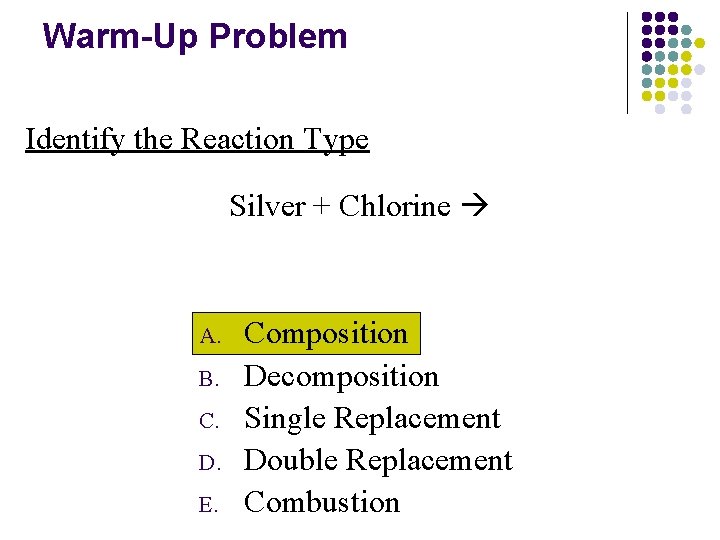
Warm-Up Problem Identify the Reaction Type Silver + Chlorine A. B. C. D. E. Composition Decomposition Single Replacement Double Replacement Combustion

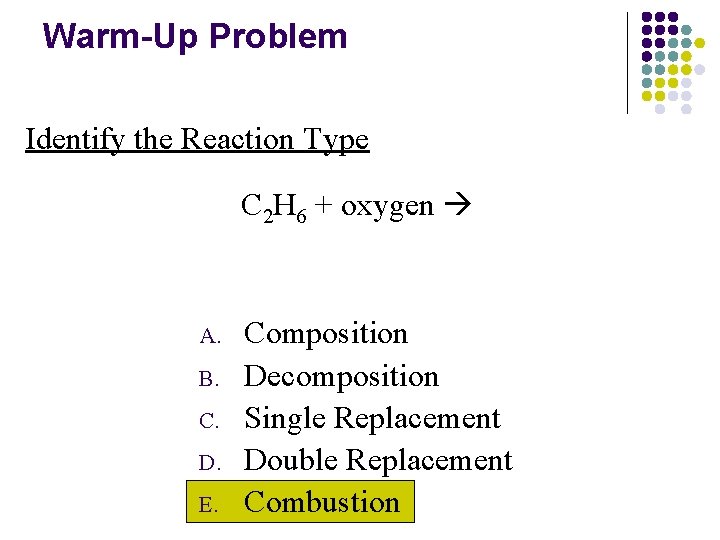
Warm-Up Problem Identify the Reaction Type C 2 H 6 + oxygen A. B. C. D. E. Composition Decomposition Single Replacement Double Replacement Combustion

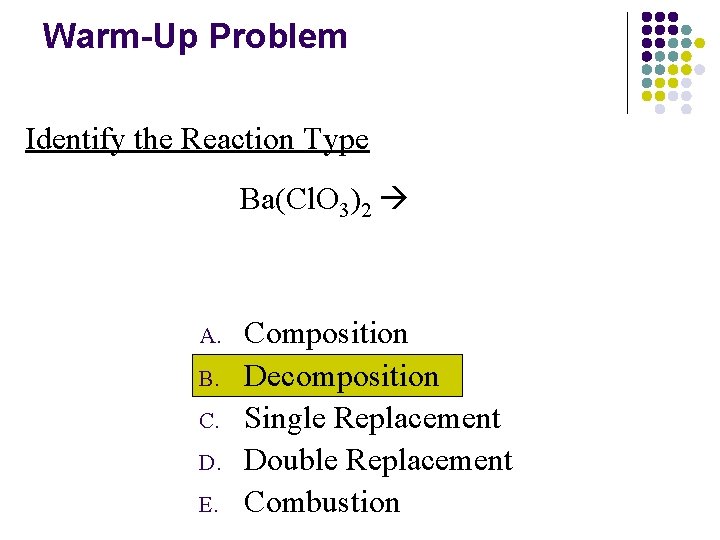
Warm-Up Problem Identify the Reaction Type Ba(Cl. O 3)2 A. B. C. D. E. Composition Decomposition Single Replacement Double Replacement Combustion

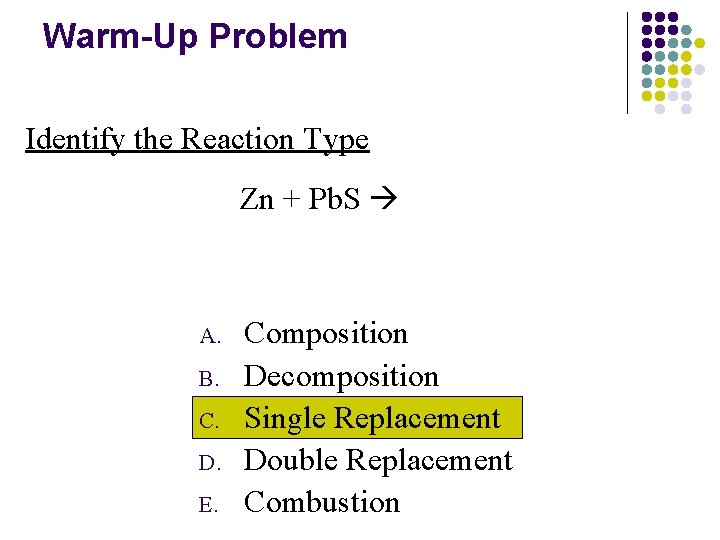
Warm-Up Problem Identify the Reaction Type Zn + Pb. S A. B. C. D. E. Composition Decomposition Single Replacement Double Replacement Combustion

SR Rxn: -- since Mg is higher than H, it reacts Tip: Use ‘nerdy name’ for hydrochloric acid Reminder: Hydrogen is Diatomic, so formula is H 2 Problem hydrogen chloride#1 + magnesium chloride + hydrogen (g) 2 HCl(aq) + Mg(s) Mg. Cl 2(aq) + H 2(g) Which would be the result of combining hydrochloric acid with magnesium? A. B. C. D. Nothing will happen It will form a precipitate It will bubble. It will burst into flames.

DR Rxn: -Tip: Use ‘nerdy name’ for nitric acid Reminder: Hydrogen is not on your solubility table, so look at gases & liquids below it. Problem hydrogen nitrate(aq)#2 + sodium sulfide sodium nitrate(aq) + hydrogen sulfide (g) 2 HNO 3(aq) + Na 2 S(aq) 2 Na. NO 3(aq) + H 2 S(g) Which would be the result of reacting nitric acid (aq) with sodium sulfide (aq)? A. B. C. D. Nothing will happen It will form a precipitate It will bubble. It will burst into flames.

DR Rxn: -Tip: Switch Partners and look for a precipitate, gas, or liquid Reminder: When both ‘possible products’ are soluble, it is a “No Reaction” Problem #3 lead chlorate(aq) + barium nitrate(aq) lead nitrate(aq) + barium chlorate(aq) Which would be the result of combining aqueous lead chlorate (aq) with barium nitrate? (aq) I thought this would be exciting…. A. B. C. D. Nothing will happen It will form a precipitate It will bubble. It will burst into flames.

Combustion Rxn: -Tip: Hydrocarbon + Oxygen is a combustion reaction Reminder: A hydrocarbon will contain C, H, and sometimes O Problem #4 Combustion reactions are extremely exothermic and the heat from them is used as our main source of energy Which would be the result of reacting C 3 H 7 OH with oxygen? C. Nothing will happen It will form a precipitate It will bubble. D. It will give off much energy. A. B.

DR Rxn: -Tip: Switch Partners and look for a precipitate, gas, or liquid Reminder: When one of the ‘possible products’ in Insoluble, it will be a precipitate In this case, the lead bromide will be a solid precipitate Problem #5 lead chlorate(aq) + sodium bromide(aq) lead bromide (s) + sodium chlorate(aq) Which would be the result of combining aqueous lead chlorate (aq) with sodium bromide (aq)? A. B. C. D. Nothing will happen It will form a precipitate It will bubble. It will burst into flames.

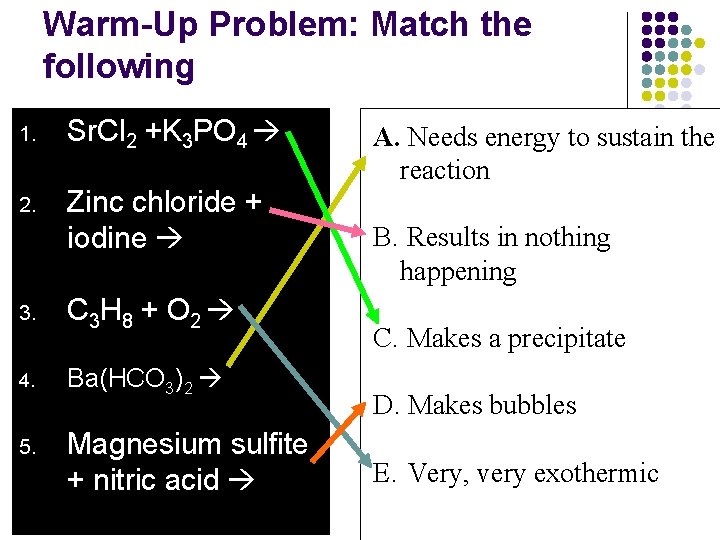
Warm-Up Problem: Match the following 1. Sr. Cl 2 +K 3 PO 4 2. Zinc chloride + iodine 3. C 3 H 8 + O 2 4. Ba(HCO 3)2 5. Magnesium sulfite + nitric acid A. Needs energy to sustain the reaction B. Results in nothing happening C. Makes a precipitate D. Makes bubbles E. Very, very exothermic

Which one does not belong? A. B. C. D. p. H = 12. 7 KOH Phenolpthalein is colorless Red litmus turns blue

Which one does not belong? A. B. C. D. p. H = 12. 7 KOH Phenolpthalein is colorless Red litmus turns blue

Which one does not belong? A. B. C. D. Reacts with metal Blue Litmus turns red Tastes sour Does not conduct electricity

Which one does not belong? A. B. C. D. Reacts with metal Blue Litmus turns red Tastes sour Does not conduct electricity

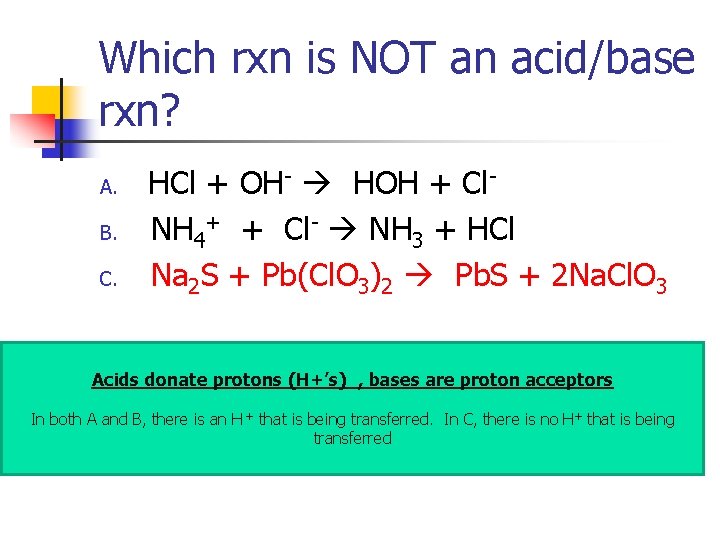
Which rxn is NOT an acid/base rxn? A. B. C. HCl + OH- HOH + Cl. NH 4+ + Cl- NH 3 + HCl Na 2 S + Pb(Cl. O 3)2 Pb. S + 2 Na. Cl. O 3

Which rxn is NOT an acid/base rxn? A. B. C. HCl + OH- HOH + Cl. NH 4+ + Cl- NH 3 + HCl Na 2 S + Pb(Cl. O 3)2 Pb. S + 2 Na. Cl. O 3 Acids donate protons (H+’s)_, bases are proton acceptors In both A and B, there is an H + that is being transferred. In C, there is no H+ that is being transferred

Which are Bases? n n n Ca(OH)2 HCl Cl. ONa. Cl Na. OH

Which are Bases? n n n Ca(OH)2 HCl Cl. O- (negative charge attracts proton) Na. Cl Na. OH

Label A thru E on whiteboard and write True or False for each True or False… Acid Rain. . A. B. C. D. E. Has a p. H of less than 5. 6 Is caused by volcanoes and lightning Will burn your skin Contains nitric and sulfuric acid Is formed by a decomposition reaction

True or False… Acid Rain. . A. B. C. D. E. Has a p. H of 5. 6 or less -- True Is caused by volcanoes and lightning –False – H 2 CO 3 not main contributor Will burn your skin -- False Contains nitric and sulfuric acid -True Is formed by a decomposition reaction -- False (formed by Composition #5)

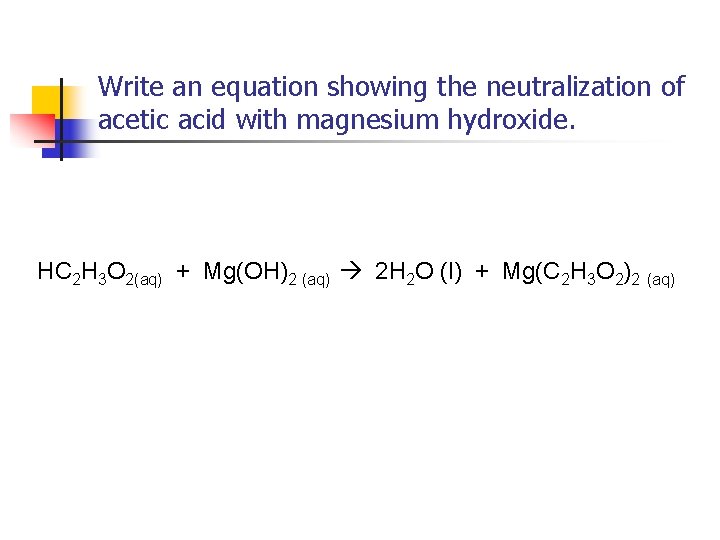
Write an equation showing the neutralization of acetic acid with magnesium hydroxide.

Write an equation showing the neutralization of acetic acid with magnesium hydroxide. HC 2 H 3 O 2(aq) + Mg(OH)2 (aq) 2 H 2 O (l) + Mg(C 2 H 3 O 2)2 (aq)

What causes acid rain? Burning Fossil Fuels release NOx and SOx gases which combine chemically with the water in the air to make nitric and sulfuric acid.

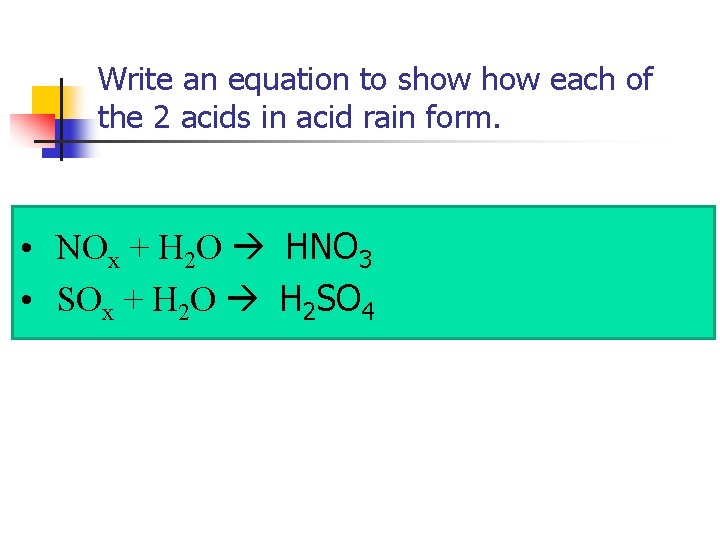
Write an equation to show each of the 2 acids in acid rain form. • NOx + H 2 O HNO 3 • SOx + H 2 O H 2 SO 4

Explain how acid rain kills fish

Explain how acid rain harms plants (2 ways)

Why is concrete/limestone not the best choice for a monument or gravestone? Concrete and Limestone are primarily made up of Calcium carbonate. Ca. CO 3 is a base that reacts with acid, so the acid rain would react with these materials and slowly wear them away,