Unit 3 Chemical Equations and Stoichiometry Balancing Equations
Unit 3: Chemical Equations and Stoichiometry Balancing Equations

Balancing Equations Remember the law of conservation of mass? • When a chemical reaction occurs, no atoms are created or just destroyed – they are just rearranged • amount of each element on the left side (reactants) must equal the amount of the same element on the right side (products) reactants products
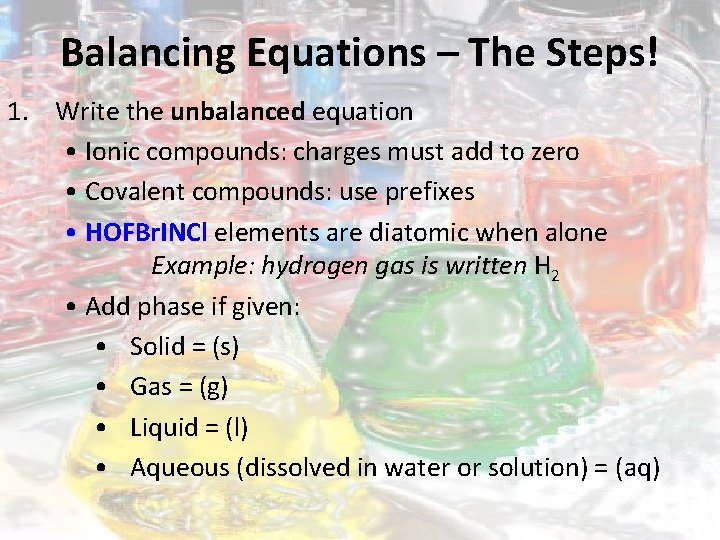
Balancing Equations – The Steps! 1. Write the unbalanced equation • Ionic compounds: charges must add to zero • Covalent compounds: use prefixes • HOFBr. INCl elements are diatomic when alone Example: hydrogen gas is written H 2 • Add phase if given: • Solid = (s) • Gas = (g) • Liquid = (l) • Aqueous (dissolved in water or solution) = (aq)

Balancing Equations Practice #1 Write and balance the following chemical equation: Lead (IV) sulfide solid + oxygen gas lead (IV) oxide solid + sulfur dioxide gas

Balancing Equations – The Steps! 2. Put coefficients in front of each substance to get the quantities on the left side = the right side 3. Use the smallest whole number coefficients possible.

Balancing Equations - Hints 1. Save “singletons” for last (elements that are not bonded with other elements) 2. Save oxygen for last (if there are no singletons) 3. Save hydrogen for 2 nd to last
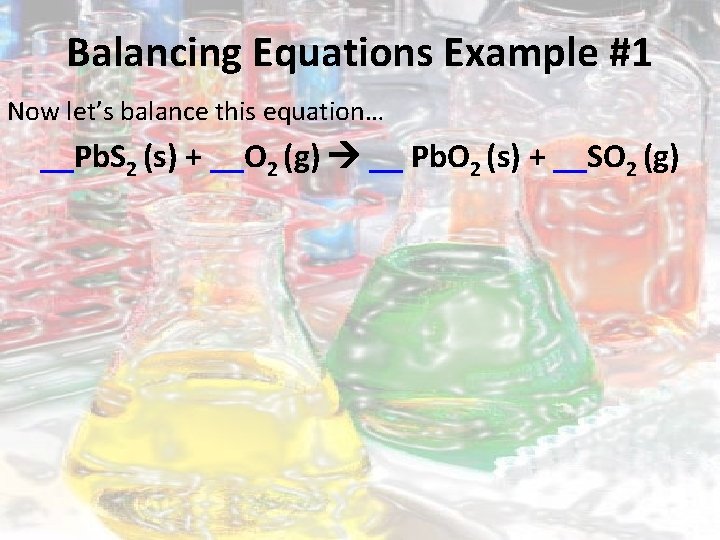
Balancing Equations Example #1 Now let’s balance this equation… __Pb. S 2 (s) + __O 2 (g) __ Pb. O 2 (s) + __SO 2 (g)
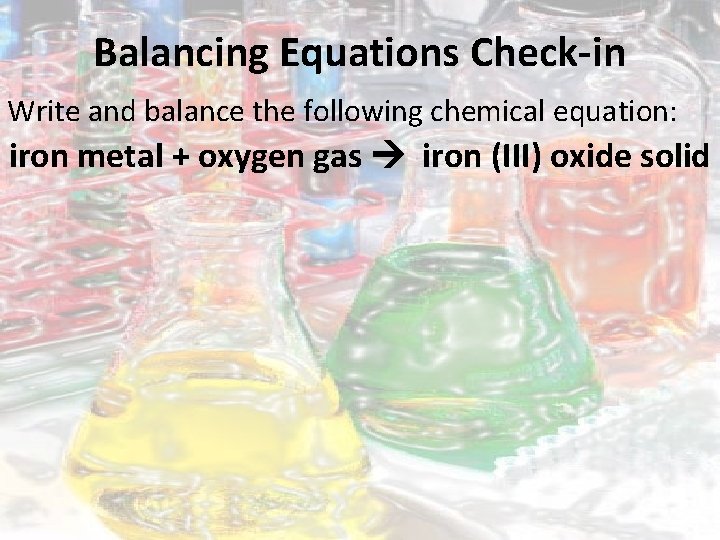
Balancing Equations Check-in Write and balance the following chemical equation: iron metal + oxygen gas iron (III) oxide solid

Balancing Equations Practice #2 Write and balance the following chemical equation: ammonium phosphate soln. + magnesium nitrate soln. ammonium nitrate soln. + magnesium phosphate solid
- Slides: 9