Unit 3 CHEMICAL BONDING Covalent Bonding Covalent Bonding

Unit 3 CHEMICAL BONDING (Covalent Bonding)
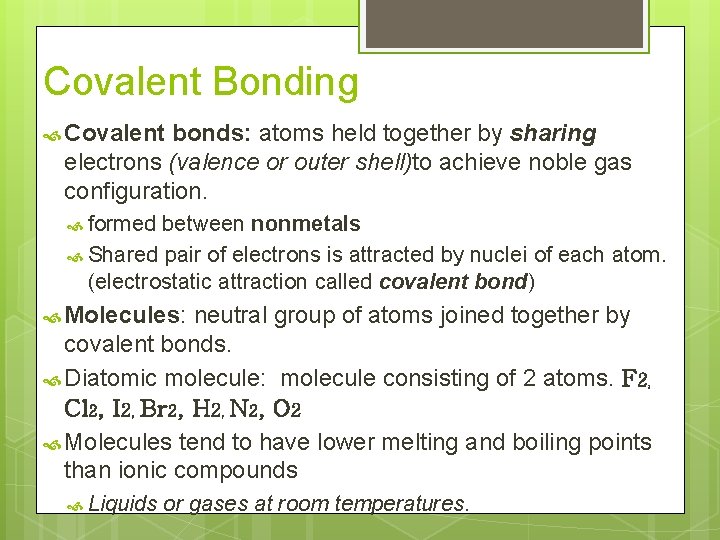
Covalent Bonding Covalent bonds: atoms held together by sharing electrons (valence or outer shell)to achieve noble gas configuration. formed between nonmetals Shared pair of electrons is attracted by nuclei of each atom. (electrostatic attraction called covalent bond) Molecules: neutral group of atoms joined together by covalent bonds. Diatomic molecule: molecule consisting of 2 atoms. F 2, Cl 2, I 2, Br 2, H 2, N 2, O 2 Molecules tend to have lower melting and boiling points than ionic compounds Liquids or gases at room temperatures.
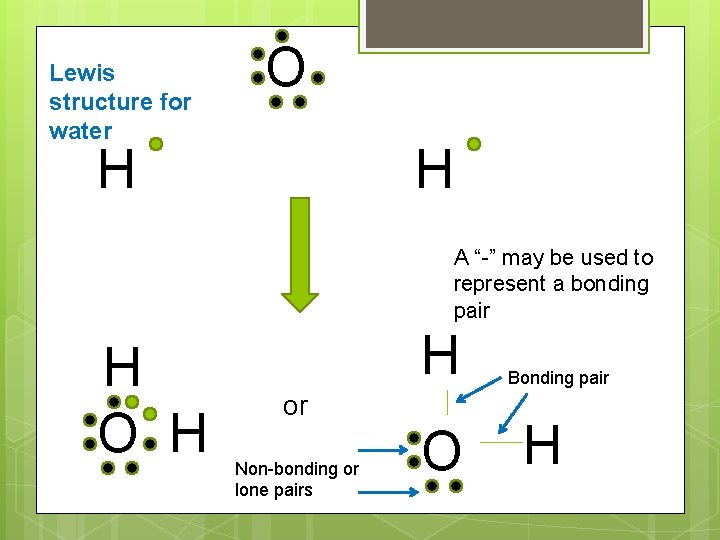
Lewis structure for water O H H A “-” may be used to represent a bonding pair H O H H or Non-bonding or lone pairs O Bonding pair H
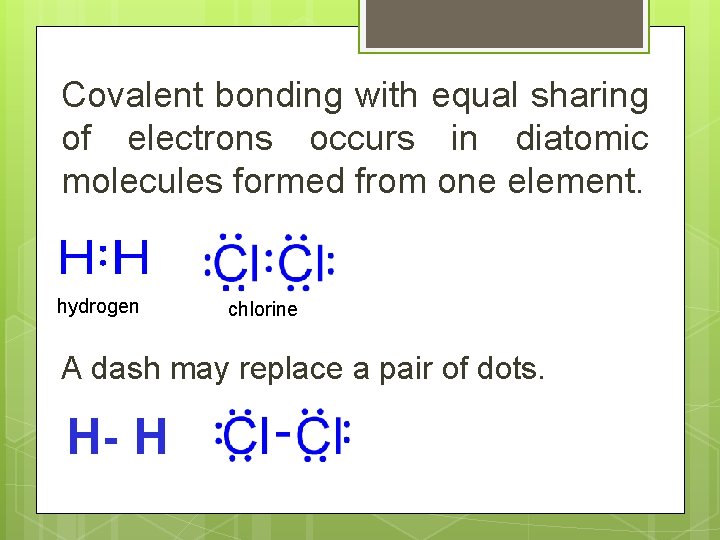
Covalent bonding with equal sharing of electrons occurs in diatomic molecules formed from one element. hydrogen chlorine A dash may replace a pair of dots. H- H
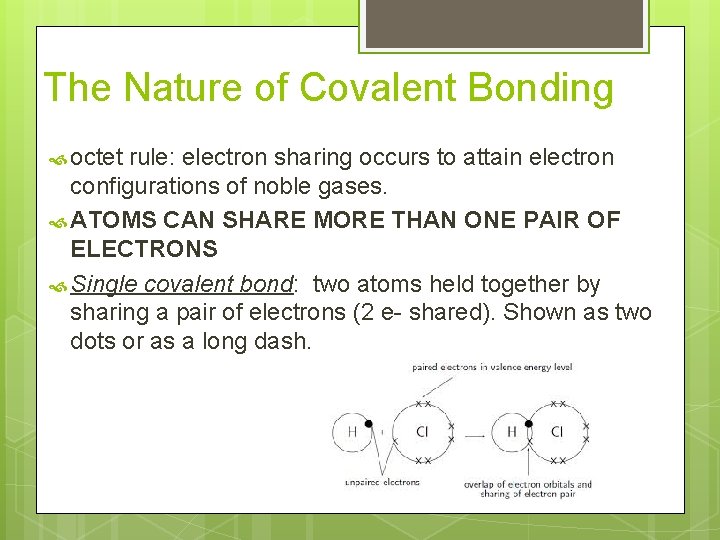
The Nature of Covalent Bonding octet rule: electron sharing occurs to attain electron configurations of noble gases. ATOMS CAN SHARE MORE THAN ONE PAIR OF ELECTRONS Single covalent bond: two atoms held together by sharing a pair of electrons (2 e- shared). Shown as two dots or as a long dash.
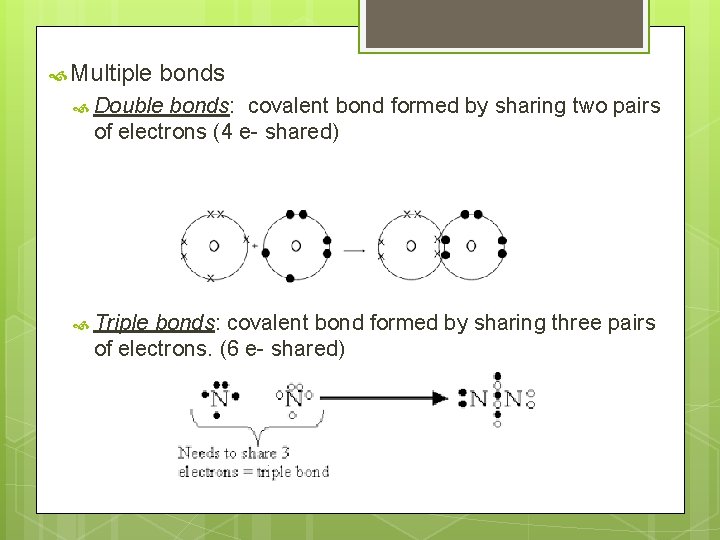
Multiple bonds Double bonds: covalent bond formed by sharing two pairs of electrons (4 e- shared) Triple bonds: covalent bond formed by sharing three pairs of electrons. (6 e- shared)
Strength of covalent bonds Bond length (distance between nuclei): the shorter the bond length, the greater the bond strength (energy required to break bond). As atomic radius increases (down a group), diatomic molecules make longer bonds (weaker bonds) Triple bond is stronger than a double bond; double bond is stronger than a single bond. Classwork Read section 8. 1 in textbook and do p 247#7 -13
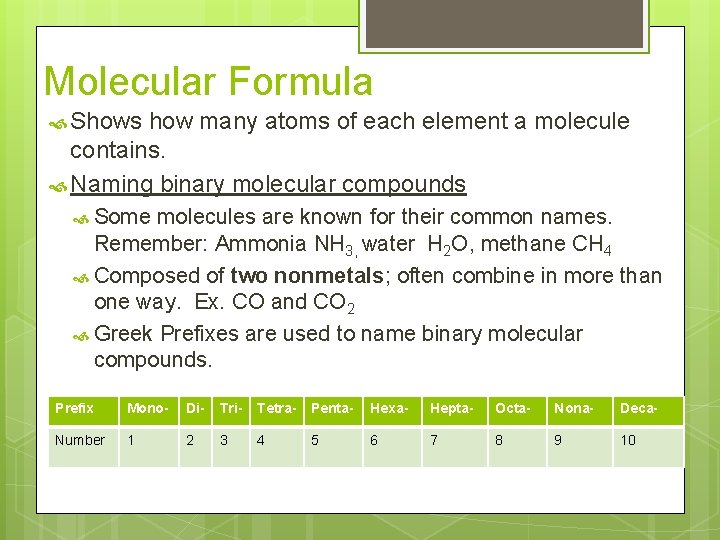
Molecular Formula Shows how many atoms of each element a molecule contains. Naming binary molecular compounds Some molecules are known for their common names. Remember: Ammonia NH 3, water H 2 O, methane CH 4 Composed of two nonmetals; often combine in more than one way. Ex. CO and CO 2 Greek Prefixes are used to name binary molecular compounds. Prefix Mono- Di- Tri- Tetra- Penta- Hexa- Hepta- Octa- Nona- Deca- Number 1 2 3 4 5 6 7 8 9 10
Nomencalture Binary Compounds Containing Two Nonmetals To name these compounds: 1. The first element keeps its name - The first element gets a prefix if it has a subscript different than 1 in the formula 2. The second element gets the –ide ending (suffix) - The second elements ALWAYS gets a prefix
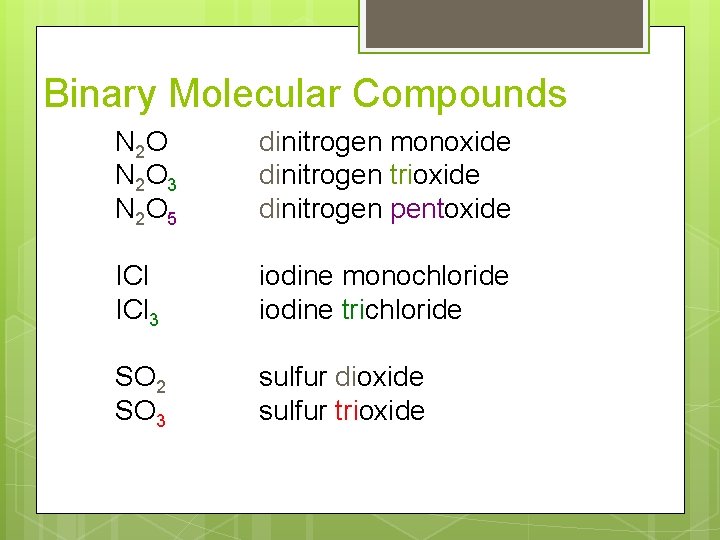
Binary Molecular Compounds N 2 O 3 N 2 O 5 dinitrogen monoxide dinitrogen trioxide dinitrogen pentoxide ICl 3 iodine monochloride iodine trichloride SO 2 SO 3 sulfur dioxide sulfur trioxide
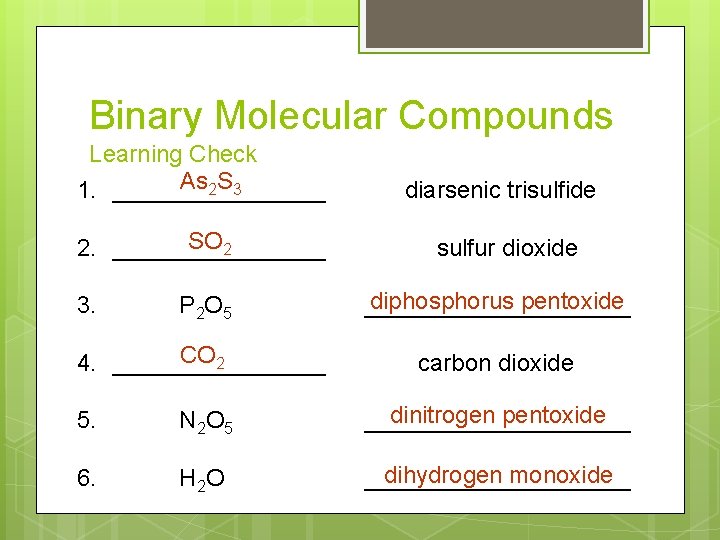
Binary Molecular Compounds Learning Check As 2 S 3 1. ________ diarsenic trisulfide SO 2 2. ________ sulfur dioxide P 2 O 5 diphosphorus pentoxide __________ CO 2 4. ________ carbon dioxide 3. 5. N 2 O 5 dinitrogen pentoxide __________ 6. H 2 O dihydrogen monoxide __________

Classwork : Handout
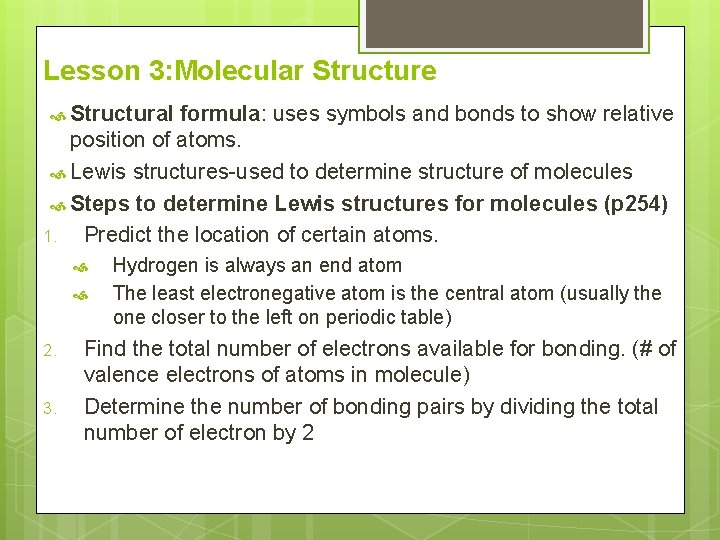
Lesson 3: Molecular Structure Structural formula: uses symbols and bonds to show relative position of atoms. Lewis structures-used to determine structure of molecules Steps to determine Lewis structures for molecules (p 254) 1. Predict the location of certain atoms. 2. 3. Hydrogen is always an end atom The least electronegative atom is the central atom (usually the one closer to the left on periodic table) Find the total number of electrons available for bonding. (# of valence electrons of atoms in molecule) Determine the number of bonding pairs by dividing the total number of electron by 2

Place one bonding pair (single bond) between central atom and terminal atoms. 5. Determine number of bonding pairs remaining: Subtract pairs used in step 4 from bonding pairs in step 3. Place lone pairs around each terminal atom bonded to the central atom to satisfy the octet rule. Any remaining pairs are assigned to the central atom. 6. Determine if central atom satisfies the octet rule. If the central atom does not have an octet, convert one or two of the lone pairs on the terminal atoms to a double or a triple bond between central and terminal atom. Some elements do not follow the octet rule: Ex. H, B, S, P, Xe 4.
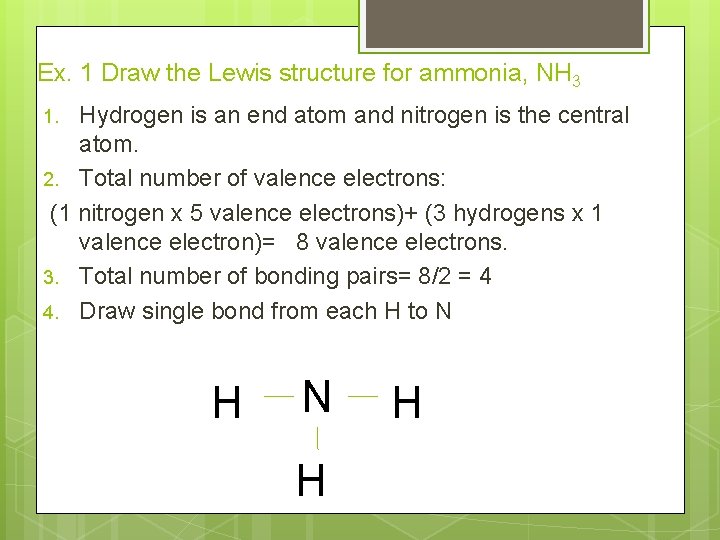
Ex. 1 Draw the Lewis structure for ammonia, NH 3 Hydrogen is an end atom and nitrogen is the central atom. 2. Total number of valence electrons: (1 nitrogen x 5 valence electrons)+ (3 hydrogens x 1 valence electron)= 8 valence electrons. 3. Total number of bonding pairs= 8/2 = 4 4. Draw single bond from each H to N 1. H N H H
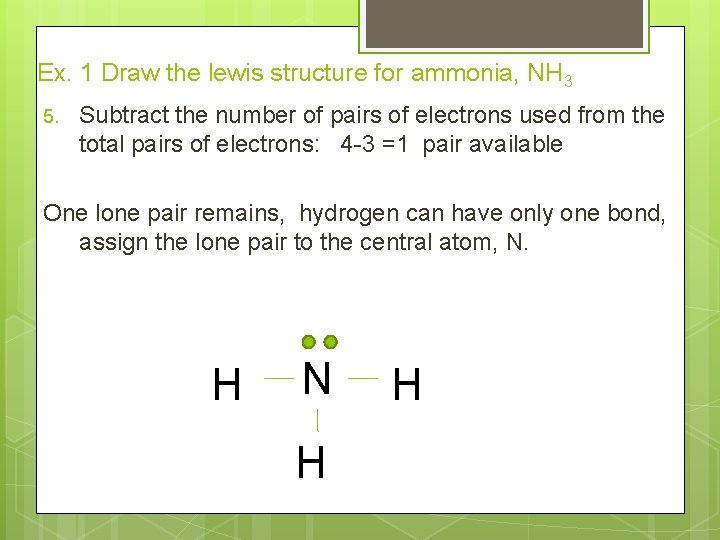
Ex. 1 Draw the lewis structure for ammonia, NH 3 5. Subtract the number of pairs of electrons used from the total pairs of electrons: 4 -3 =1 pair available One lone pair remains, hydrogen can have only one bond, assign the lone pair to the central atom, N. H N H H
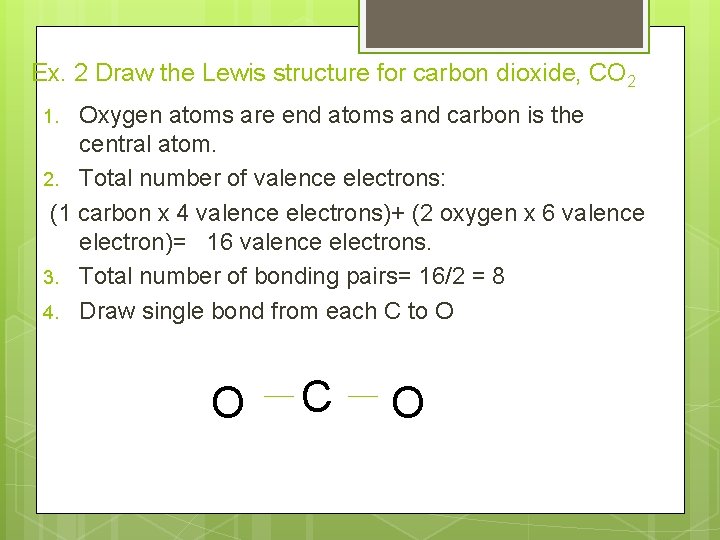
Ex. 2 Draw the Lewis structure for carbon dioxide, CO 2 Oxygen atoms are end atoms and carbon is the central atom. 2. Total number of valence electrons: (1 carbon x 4 valence electrons)+ (2 oxygen x 6 valence electron)= 16 valence electrons. 3. Total number of bonding pairs= 16/2 = 8 4. Draw single bond from each C to O 1. O C O
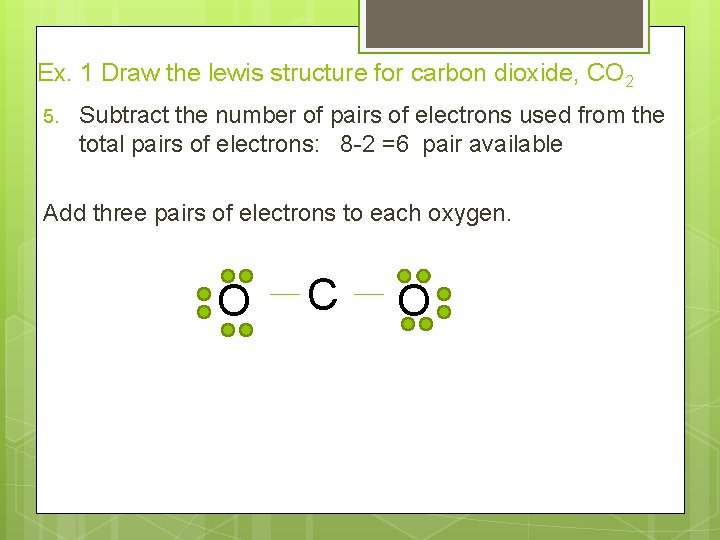
Ex. 1 Draw the lewis structure for carbon dioxide, CO 2 5. Subtract the number of pairs of electrons used from the total pairs of electrons: 8 -2 =6 pair available Add three pairs of electrons to each oxygen. O C O
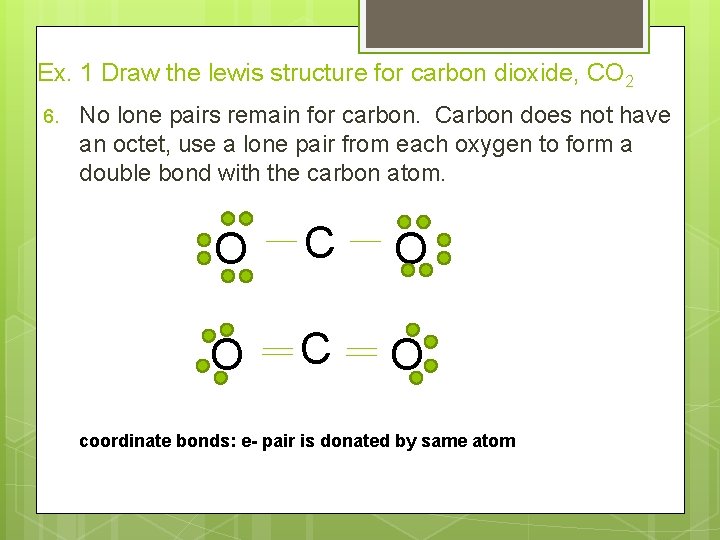
Ex. 1 Draw the lewis structure for carbon dioxide, CO 2 6. No lone pairs remain for carbon. Carbon does not have an octet, use a lone pair from each oxygen to form a double bond with the carbon atom. O C O coordinate bonds: e- pair is donated by same atom

Learning Check Draw the Lewis structure for a)Carbon monoxide, CO b)ethylene, C 2 H 4

Lewis structures for polyatomic ions The difference is calculating number of valence electrons: First find the number of valence electrons available in the atoms present in the ion. Then subtract the ion charge if the ion is positive and add the ion charge if the ion is negative
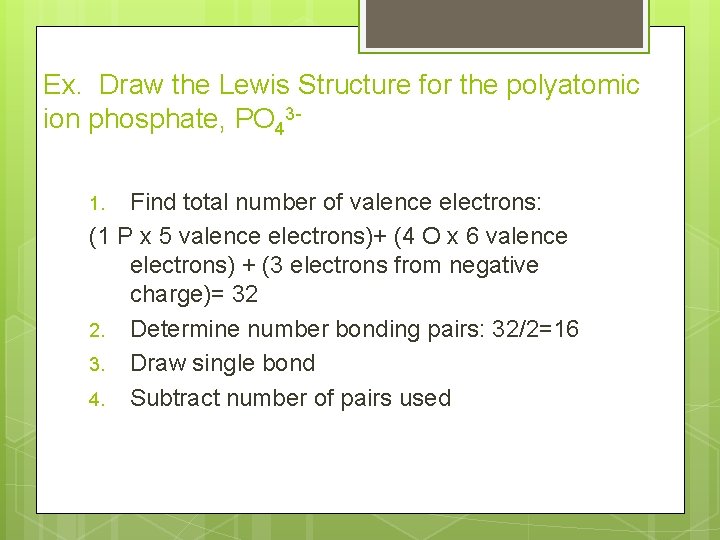
Ex. Draw the Lewis Structure for the polyatomic ion phosphate, PO 43 Find total number of valence electrons: (1 P x 5 valence electrons)+ (4 O x 6 valence electrons) + (3 electrons from negative charge)= 32 2. Determine number bonding pairs: 32/2=16 3. Draw single bond 4. Subtract number of pairs used 1.

Resonance structures and exceptions to octet rule �Resonance: Occurs when more than one valid Lewis structure can be written for a molecule or ion. �Differ in the position of electron pairs �Ex. O 3 , SO 2
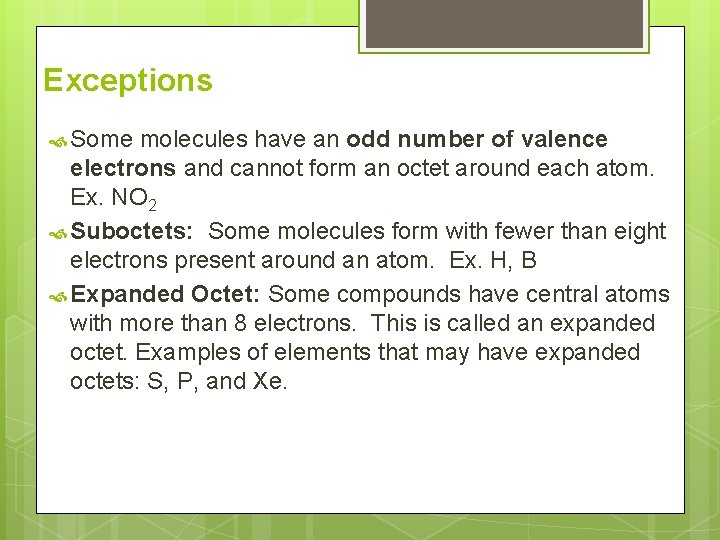
Exceptions Some molecules have an odd number of valence electrons and cannot form an octet around each atom. Ex. NO 2 Suboctets: Some molecules form with fewer than eight electrons present around an atom. Ex. H, B Expanded Octet: Some compounds have central atoms with more than 8 electrons. This is called an expanded octet. Examples of elements that may have expanded octets: S, P, and Xe.
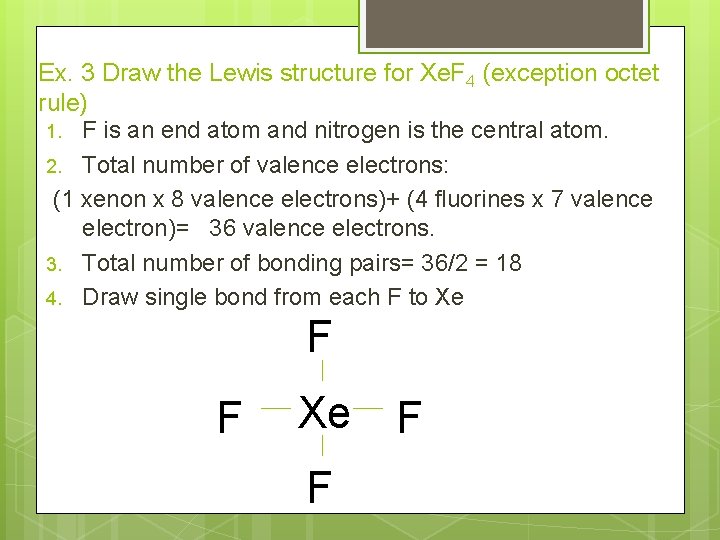
Ex. 3 Draw the Lewis structure for Xe. F 4 (exception octet rule) 1. F is an end atom and nitrogen is the central atom. 2. Total number of valence electrons: (1 xenon x 8 valence electrons)+ (4 fluorines x 7 valence electron)= 36 valence electrons. 3. Total number of bonding pairs= 36/2 = 18 4. Draw single bond from each F to Xe F F
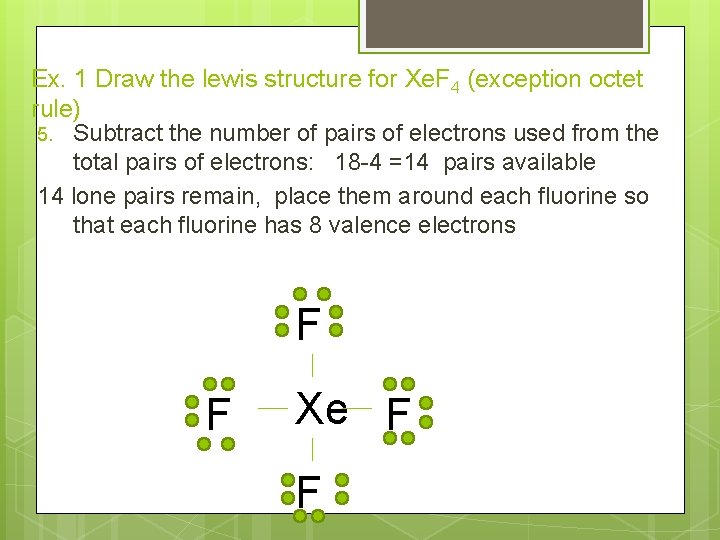
Ex. 1 Draw the lewis structure for Xe. F 4 (exception octet rule) 5. Subtract the number of pairs of electrons used from the total pairs of electrons: 18 -4 =14 pairs available 14 lone pairs remain, place them around each fluorine so that each fluorine has 8 valence electrons F F Xe F F
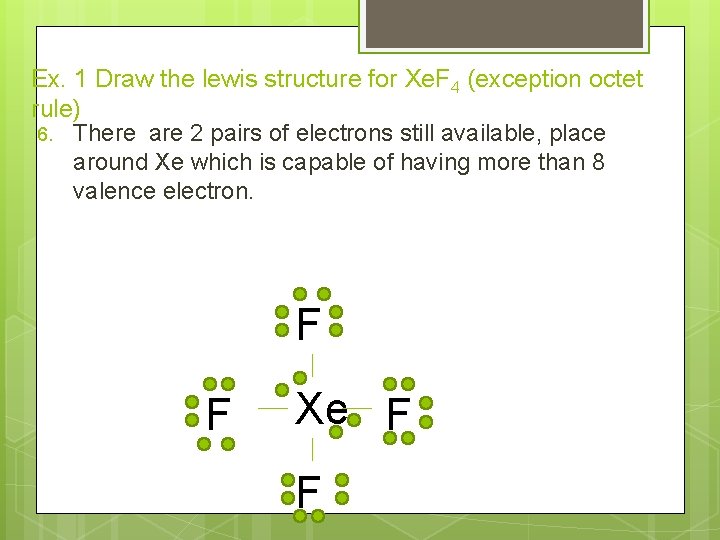
Ex. 1 Draw the lewis structure for Xe. F 4 (exception octet rule) 6. There are 2 pairs of electrons still available, place around Xe which is capable of having more than 8 valence electron. F F Xe F F

Cw lewis structure handout
Molecular Shape VSEPR (Valence shell electron pair repulsion) Model repulsion between electron pairs in a molecule result in atoms existing at fixed angles from each other. (Remember balloon activity) Bond angle: angle formed between two end atoms and the central atom Shared electron pairs repel each other A greater repulsion occurs between unshared electron pairs (occupy larger orbital) and shared electron pairs. VSEPR Theory Read p 262 connection to biology.
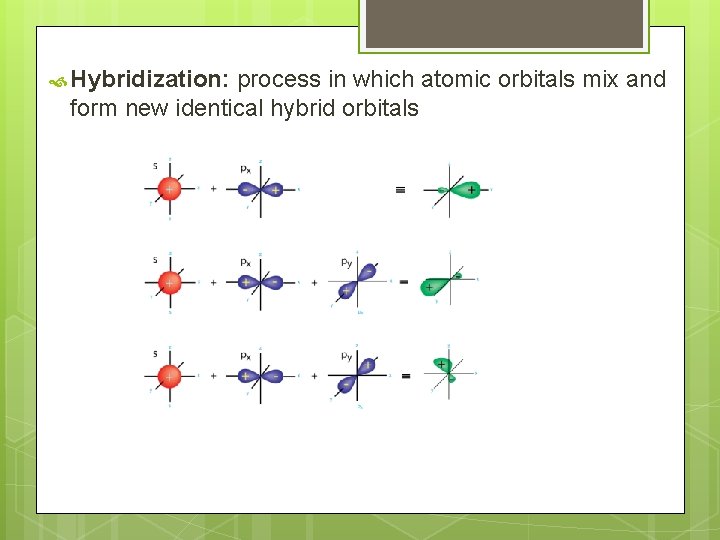
Hybridization: process in which atomic orbitals mix and form new identical hybrid orbitals
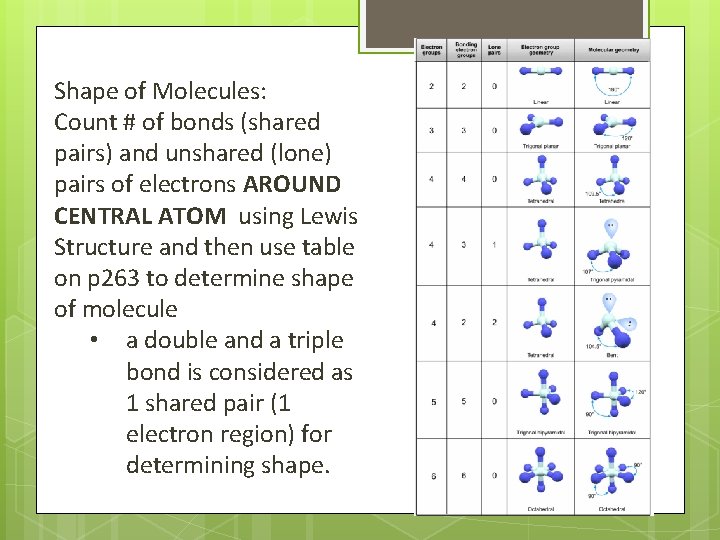
Shape of Molecules: Count # of bonds (shared pairs) and unshared (lone) pairs of electrons AROUND CENTRAL ATOM using Lewis Structure and then use table on p 263 to determine shape of molecule • a double and a triple bond is considered as 1 shared pair (1 electron region) for determining shape.
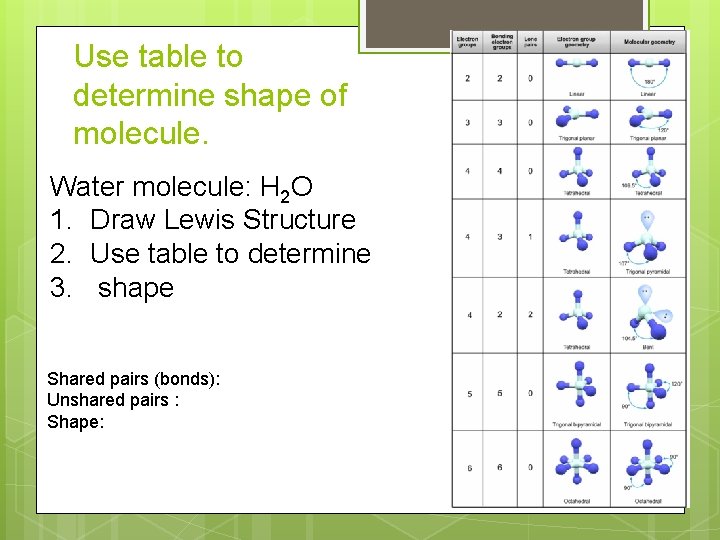
Use table to determine shape of molecule. Water molecule: H 2 O 1. Draw Lewis Structure 2. Use table to determine 3. shape Shared pairs (bonds): Unshared pairs : Shape: SO 2
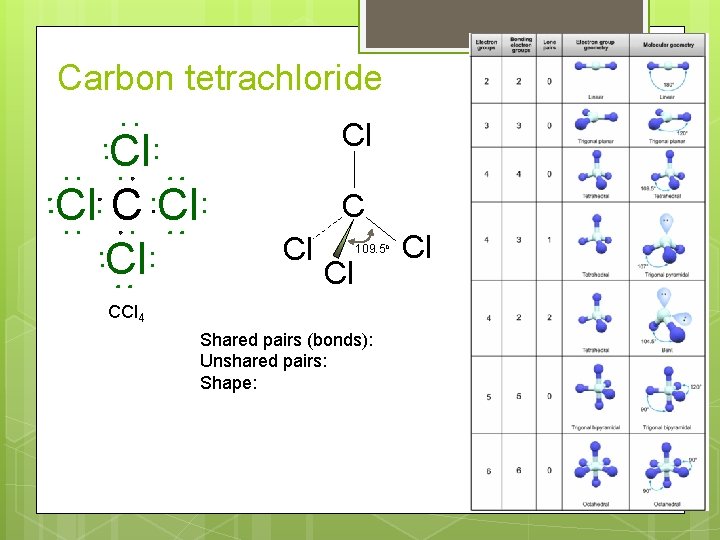
Carbon tetrachloride Cl Cl Cl 109. 5 o CCl 4 Shared pairs (bonds): Unshared pairs: Shape: Cl

Classwork: p 264 #56 - 67
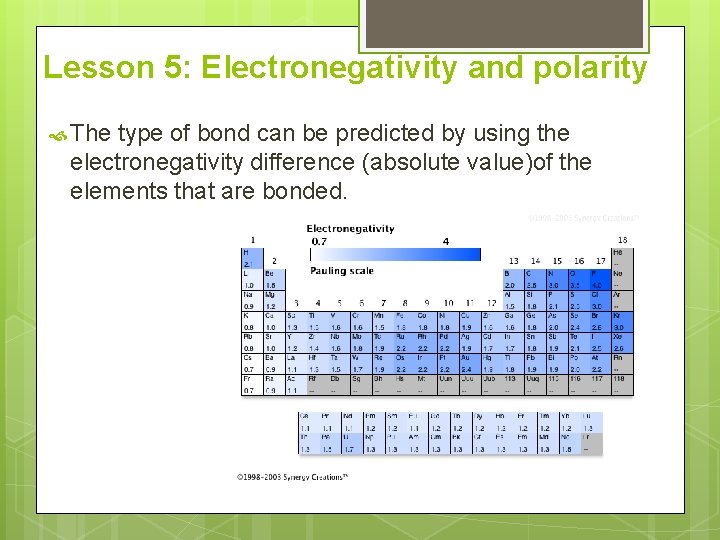
Lesson 5: Electronegativity and polarity The type of bond can be predicted by using the electronegativity difference (absolute value)of the elements that are bonded.
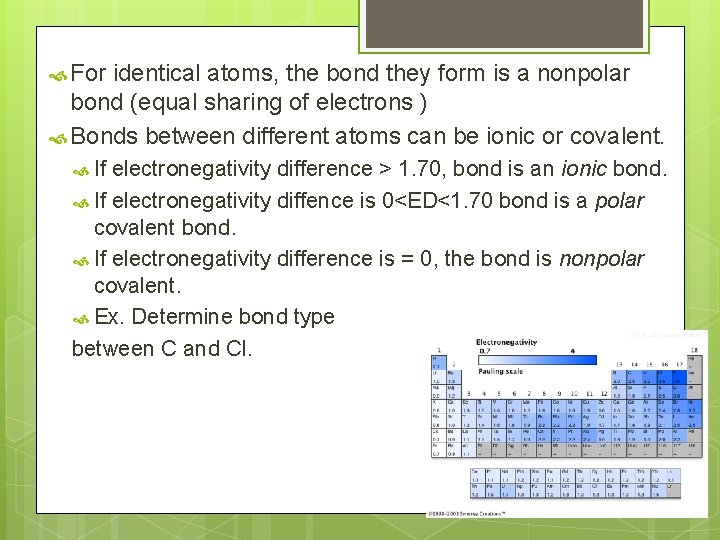
For identical atoms, the bond they form is a nonpolar bond (equal sharing of electrons ) Bonds between different atoms can be ionic or covalent. If electronegativity difference > 1. 70, bond is an ionic bond. If electronegativity diffence is 0<ED<1. 70 bond is a polar covalent bond. If electronegativity difference is = 0, the bond is nonpolar covalent. Ex. Determine bond type between C and Cl.
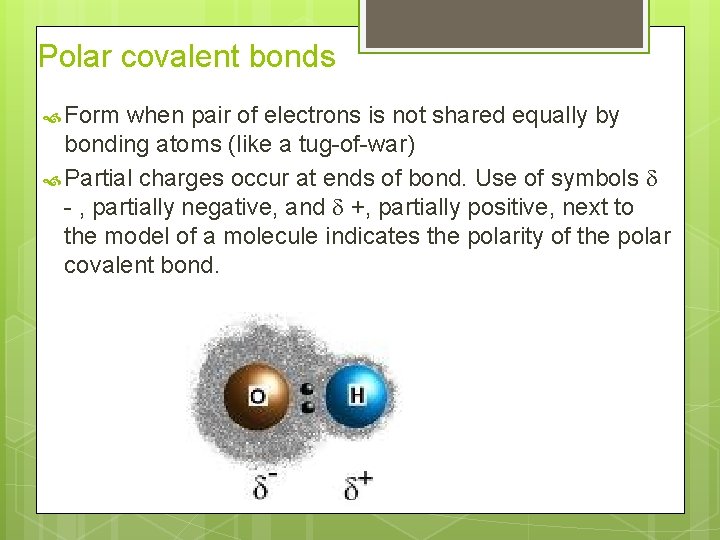
Polar covalent bonds Form when pair of electrons is not shared equally by bonding atoms (like a tug-of-war) Partial charges occur at ends of bond. Use of symbols - , partially negative, and +, partially positive, next to the model of a molecule indicates the polarity of the polar covalent bond.
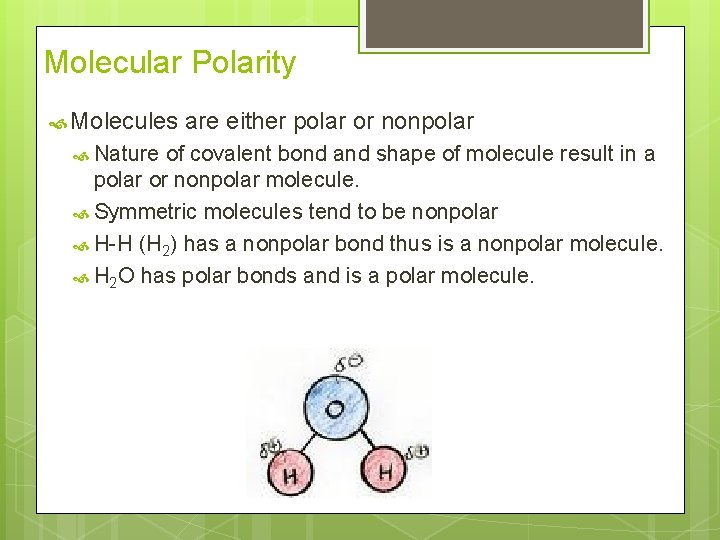
Molecular Polarity Molecules Nature are either polar or nonpolar of covalent bond and shape of molecule result in a polar or nonpolar molecule. Symmetric molecules tend to be nonpolar H-H (H 2) has a nonpolar bond thus is a nonpolar molecule. H 2 O has polar bonds and is a polar molecule.
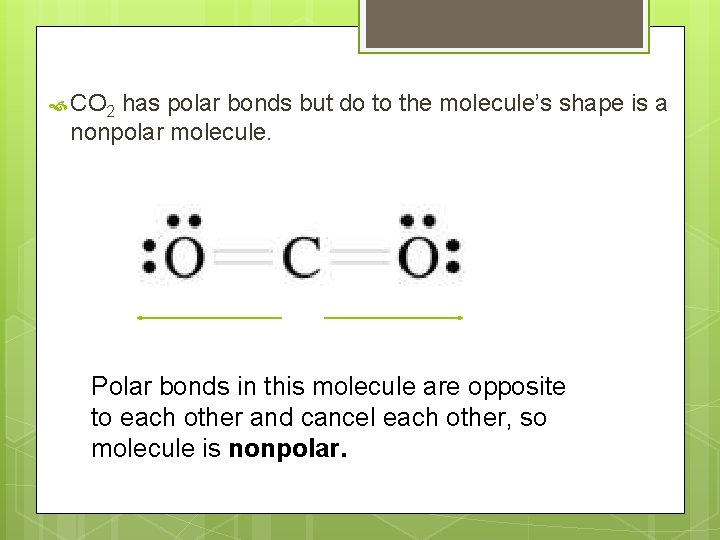
CO 2 has polar bonds but do to the molecule’s shape is a nonpolar molecule. Polar bonds in this molecule are opposite to each other and cancel each other, so molecule is nonpolar.
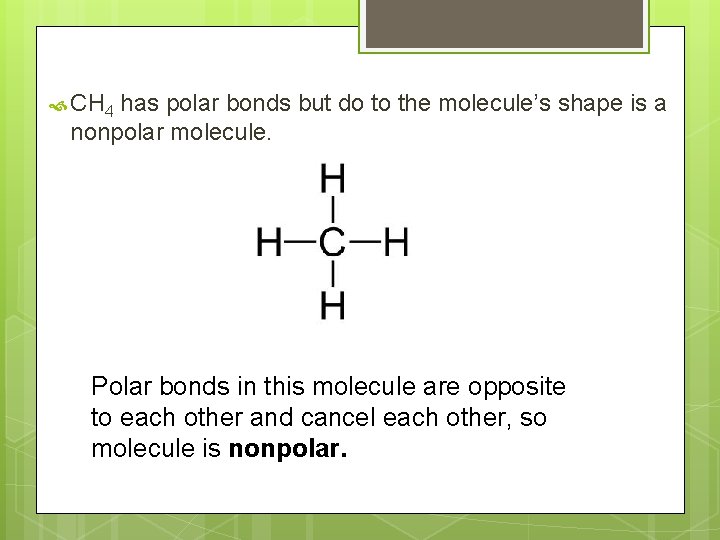
CH 4 has polar bonds but do to the molecule’s shape is a nonpolar molecule. Polar bonds in this molecule are opposite to each other and cancel each other, so molecule is nonpolar.
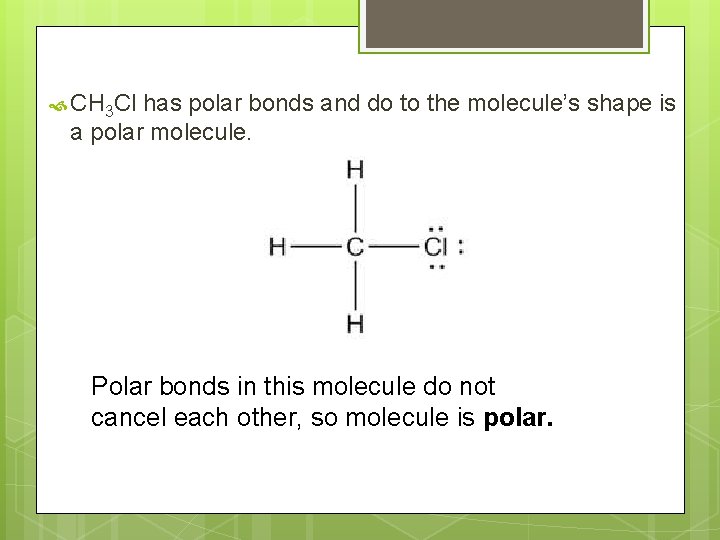
CH 3 Cl has polar bonds and do to the molecule’s shape is a polar molecule. Polar bonds in this molecule do not cancel each other, so molecule is polar.
Solubility of molecules (ability to dissolve) Polar molecules and ionic compounds are usually soluble in polar substances. Nonpolar molecules dissolve in nonpolar substance. Intermolecular Weak forces (or Van der Waals forces): attraction forces between molecules. Dispersion force: weak force between nonpolar molecules Dipole-dipole: force between oppositely charged ends of two polar molecules Hydrogen bond: strong force, forms between the hydrogen end of one dipole and a F, O, or N atom on another dipole.

CW p 275 #113 -122 except 116
- Slides: 43